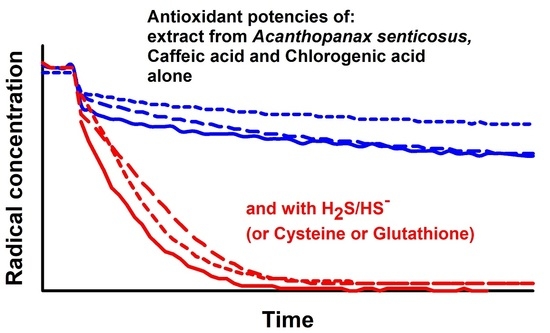
Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA
Abstract
:1. Introduction
2. Results
2.1. Experiments Using ASRE
2.1.1. ASRE Interacting with H2S Reduces ●cPTIO
2.1.2. ASRE Interacting with Cys or GSH (But Not with GSSG, MET or Cystine) Reduces ●cPTIO
2.1.3. Effect of ASRE on Na2S4 Induced Reduction of ●cPTIO
2.1.4. Effect of ASRE on Its Own and in the Mixture with H2S, Polysulfides, Cys and GSH on pDNA Cleavage
2.2. Experiments Using ASRE Components: CA, CGA, Protocatechuic Acid (PCA), Eleutheroside B (EB) and Eleutheroside E (EE)
2.2.1. CA, CGA and PCA (But Not EB or EE) Interacting with H2S Reduce ●cPTIO
2.2.2. CA, CGA, PCA, EB or EE Interacting with Polysulfide Do Not Reduce ●cPTIO
2.2.3. CA, CGA and PCA (But Not EB or EE) in the Mixture with Cys or GSH Reduce ●cPTIO
2.2.4. Effect of the ASRE Components on pDNA Cleavage
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Preparation of Aqueous Root Extract from Acanthopanax Senticosus (ASRE)
4.3. UV-VIS of ●cPTIO Radical
4.4. Plasmid DNA Cleavage
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Zhang, Y.; Gao, H.; Zhang, Z.; Zhang, Y.; Wang, Z.; Zhang, J.; Deng, B.; Qiu, Z.; Fu, C. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2021, 268, 113586. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, C.; Leng, A.; Qu, J. Advances in the Extraction, Purification, Structural Characteristics and Biological Activities of Eleutherococcus senticosus Polysaccharides: A Promising Medicinal and Edible Resource with Development Value. Front. Pharmacol. 2021, 12, 753007. [Google Scholar] [CrossRef] [PubMed]
- Vaško, L.; Vašková, J.; Fejerčáková, A.; Mojžišová, G.; Poráčová, J. Comparison of some antioxidant properties of plant extracts from Origanum vulgare, Salvia officinalis, Eleutherococcus senticosus and Stevia rebaudiana. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 614–622. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, M.L.; Kim, D.B.; Shin, G.H.; Lee, J.H.; Lee, J.S.; Park, S.O.; Lee, S.J.; Shin, H.M.; Lee, O.H. The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef]
- Załuski, D.; Kuźniewski, R.; Janeczko, Z. HPTLC-profiling of eleutherosides, mechanism of antioxidative action of eleutheroside E1, the PAMPA test with LC/MS detection and the structure-activity relationship. Saudi J. Biol. Sci. 2018, 25, 520–528. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Song, C.; Li, S.; Duan, F.; Liu, M.; Shan, S.; Ju, T.; Zhang, Y.; Lu, W. The Therapeutic Effect of Acanthopanax senticosus Components on Radiation-Induced Brain Injury Based on the Pharmacokinetics and Neurotransmitters. Molecules 2022, 27, 1106. [Google Scholar] [CrossRef]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022. Ahead of print. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative mechanisms in chlorogenic acid. Food Chem. 2017, 237, 390–398. [Google Scholar] [CrossRef]
- Hu, Z.F.; Yu, W.L.; Zhao, Y.P. Study on the Scavenging of ROS and Anti-lipid Peroxidation by Chlorogenic Acid. Food Sci. 2006, 27, 128–130. [Google Scholar]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Mori, H.; Iwahashi, H. Antioxidant activity of caffeic acid through a novel mechanism under UVA irradiation. J. Clin. Biochem. Nutr. 2009, 45, 49–55. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Nael, M.A.; Elokely, K.M.; Doiron, J.A.; Leblanc, L.M.; Lassalle-Claux, G.; Salla, M.; Aldawsari, F.S.; Touaibia, M.; Vasantha Rupasinghe, H.P. Ketone Analog of Caffeic Acid Phenethyl Ester Exhibits Antioxidant Activity via Activation of ERK-Dependent Nrf2 Pathway. Appl. Sci. 2022, 12, 3062. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Zou, B.; Shen, Y.; Li, Y.; Zhang, A.; Fu, G. Molecular mechanism underlying the ability of caffeic acid to decrease uric acid levels in hyperuricemia rats. J. Funct. Foods 2019, 57, 150–156. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides-a new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues and organs. Physiol. Rev. 2022. Ahead of print. [Google Scholar] [CrossRef]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kuhnle, G.G.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, E4651–E4660. [Google Scholar] [CrossRef]
- Abiko, Y.; Shinkai, Y.; Unoki, T.; Hirose, R.; Uehara, T.; Kumagai, Y. Polysulfide Na2S4 regulates the activation of PTEN/Akt/CREB signaling and cytotoxicity mediated by 1,4-naphthoquinone through formation of sulfur adducts. Sci. Rep. 2017, 7, 4814. [Google Scholar] [CrossRef]
- Misak, A.; Grman, M.; Bacova, Z.; Rezuchova, I.; Hudecova, S.; Ondriasova, E.; Krizanova, O.; Brezova, V.; Chovanec, M.; Ondrias, K. Polysulfides and products of H2S/S-nitrosoglutathione in comparison to H2S, glutathione and antioxidant Trolox are potent scavengers of superoxide anion radical and produce hydroxyl radical by decomposition of H2O2. Nitric Oxide 2018, 76, 136–151. [Google Scholar] [CrossRef]
- Grman, M.; Misak, A.; Kurakova, L.; Brezova, V.; Cacanyiova, S.; Berenyiova, A.; Balis, P.; Tomasova, L.; Kharma, A.; Domínguez-Álvarez, E.; et al. Products of Sulfide/Selenite Interaction Possess Antioxidant Properties, Scavenge Superoxide-Derived Radicals, React with DNA, and Modulate Blood Pressure and Tension of Isolated Thoracic Aorta. Oxid. Med. Cell. Longev. 2019, 2019, 9847650. [Google Scholar] [CrossRef]
- Kharma, A.; Grman, M.; Misak, A.; Domínguez-Álvarez, E.; Nasim, M.J.; Ondrias, K.; Chovanec, M.; Jacob, C. Inorganic Polysulfides and Related Reactive Sulfur–Selenium Species from the Perspective of Chemistry. Molecules 2019, 24, 1359. [Google Scholar] [CrossRef]
- Misak, A.; Kurakova, L.; Goffa, E.; Brezova, V.; Grman, M.; Ondriasova, E.; Chovanec, M.; Ondrias, K. Sulfide (Na2S) and Polysulfide (Na2S2) Interacting with Doxycycline Produce/Scavenge Superoxide and Hydroxyl Radicals and Induce/Inhibit DNA Cleavage. Molecules 2019, 24, 1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.; Webb, G.D.; Bian, J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Ueland, P.M.; Refsum, H.; Graham, I.M.; Vollset, S.E. Plasma total cysteine as a risk factor for vascular disease: The European concerted action project. Circulation 2001, 103, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Delaunay-Moisan, A.; Ponsero, A.; Toledano, M.B. Reexamining the Function of Glutathione in Oxidative Protein Folding and Secretion. Antioxid. Redox Signal. 2017, 27, 1178–1199. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Mody, V.C.; Cai, J.; Lynn, M.J.; Sternberg, P. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000, 28, 625–635. [Google Scholar] [CrossRef]
- Jonas, C.R.; Ziegler, T.R.; Gu, L.H.; Jones, D.P. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic. Biol. Med. 2002, 33, 1499–1506. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar] [CrossRef]
- Samuni, U.; Samuni, Y.; Goldstein, S. On the distinction between nitroxyl and nitric oxide using nitronyl nitroxides. J. Am. Chem. Soc. 2010, 132, 8428–8432. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misak, A.; Grman, M.; Tomasova, L.; Makara, O.; Chovanec, M.; Ondrias, K. Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA. Molecules 2022, 27, 5735. https://doi.org/10.3390/molecules27175735
Misak A, Grman M, Tomasova L, Makara O, Chovanec M, Ondrias K. Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA. Molecules. 2022; 27(17):5735. https://doi.org/10.3390/molecules27175735
Chicago/Turabian StyleMisak, Anton, Marian Grman, Lenka Tomasova, Ondrej Makara, Miroslav Chovanec, and Karol Ondrias. 2022. "Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA" Molecules 27, no. 17: 5735. https://doi.org/10.3390/molecules27175735
APA StyleMisak, A., Grman, M., Tomasova, L., Makara, O., Chovanec, M., & Ondrias, K. (2022). Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA. Molecules, 27(17), 5735. https://doi.org/10.3390/molecules27175735