Supercritical CO2 Extraction of High-Added Value Compounds from Chlorella vulgaris: Experimental Design, Modelling and Optimization
Abstract
:1. Introduction
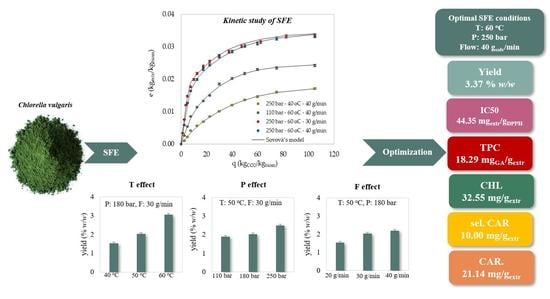
2. Results & Discussion
2.1. SFE of Bioactive Compounds
2.1.1. Effect of Pressure
2.1.2. Effect of Temperature
2.1.3. Effect of Solvent’s Flow Rate
2.1.4. Synergistic Effect
2.2. Statistical Analysis & Process Optimization
2.2.1. Regression & Reliability Analysis
2.2.2. Response Surface Plots
2.2.3. SFE Parameter Optimization
2.3. Kinetic Modelling of SFE
2.4. SFE versus SLE
2.5. Effect of Cosolvent
3. Materials and Methods
3.1. Materials
3.2. Biomass Extraction
3.2.1. Supercritical Fluid Extraction (SFE) with CO2
3.2.2. Solid-Liquid Extraction (SLE) with aq. Ethanol 90% v/v
3.3. Extract Characterization
3.3.1. Spectrophotometric Assays
3.3.2. Reversed-Phase-High Performance Liquid Chromatography (RP-HPLC)
3.4. Experimental Design, Statistical Analysis and Optimization
3.5. Mathematical Model of Extraction Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khan, M.I.; Shin, J.H.; Kim, J.D.; Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Bong, J.T.; Loy, A.C.M.; Chin, B.L.F.; Lam, M.K.; Tang, D.K.H.; Lim, H.Y.; Chai, Y.H.; Yusup, S. Artificial neural network approach for co-pyrolysis of Chlorella vulgaris and peanut shell binary mixtures using microalgae ash catalyst. Energy 2020, 207, 118289. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.; Pagès, T.; Viscor, G.; Torres, J. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical Water Technology for Extraction of Phenolic Compounds from Chlorella sp. Microalgae and Assessment on Its Antioxidant Activity. Molecules 2017, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kose, A.; Ozen, M.; Elibol, M.; Oncel, S.S. Investigation of in vitro digestibility of dietary microalga Chlorella vulgaris and cyanobacterium Spirulina platensis as a nutritional supplement. 3 Biotech. 2017, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of Pressurized Liquid Extraction of Carotenoids and Chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Kitada, K.; Machmudah, S.; Sasaki, M.; Goto, M.; Nakashima, Y.; Kumamoto, S.; Hasegawa, T. Supercritical CO2 extraction of The authors deleted the text before the words.pigment components with pharmaceutical importance from Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2009, 84, 657–661. [Google Scholar] [CrossRef]
- Li, H.-B.; Jiang, Y.; Chen, F. Isolation and Purification of Lutein from the Microalga Chlorella vulgaris by Extraction after Saponification. J. Agric. Food Chem. 2002, 50, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Palavra, A.; Coelho, J.; Barroso, J.; Rauter, A.; Fareleira, J.; Mainar, A.; Urieta, J.; Nobre, B.; Gouveia, L.; Mendes, R.; et al. Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J. Supercrit. Fluids 2011, 60, 21–27. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Shotipruk, A.; Pavasant, P.; Machmudah, S.; Goto, M. Selective Extraction of Lutein from Alcohol Treated Chlorella vulgaris by Supercritical CO2. Chem. Eng. Technol. 2012, 35, 255–260. [Google Scholar] [CrossRef]
- Gouveia, L.; Nobre, B.; Marcelo, F.; Mrejen, S.; Cardoso, M.; Palavra, A.; Mendes, R. Functional food oil coloured by pigments extracted from microalgae with supercritical CO2. Food Chem. 2007, 101, 717–723. [Google Scholar] [CrossRef]
- Cha, K.H.; Kang, S.W.; Kim, C.Y.; Um, B.H.; Na, Y.R.; Pan, C.-H. Effect of Pressurized Liquids on Extraction of Antioxidants from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 4756–4761. [Google Scholar] [CrossRef]
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and Technological Applications—A Review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Bahadar, A.; Khan, M.B.; Willmann, J.C. Accelerated production and analysis of biofuel derived from photobioreactor engineered microalgae using super critical fluid extraction. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1132–1139. [Google Scholar] [CrossRef]
- Dejoye, C.; Vian, M.A.; Lumia, G.; Bouscarle, C.; Charton, F.; Chemat, F. Combined Extraction Processes of Lipid from Chlorella vulgaris Microalgae: Microwave Prior to Supercritical Carbon Dioxide Extraction. Int. J. Mol. Sci. 2011, 12, 9332–9341. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Mendes, R.L.; Coelho, J.P.; Fernandes, H.L.; Marrucho, I.J.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Applications of supercritical CO2 extraction to microalgae and plants. J. Chem. Technol. Biotechnol. 1995, 62, 53–59. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Moradi-Kheibari, N.; Ahmadzadeh, H. Supercritical carbon dioxide extraction and analysis of lipids from Chlorella vulgaris using gas chromatography. J. Iran. Chem. Soc. 2017, 14, 2427–2436. [Google Scholar] [CrossRef]
- Obeid, S.; Beaufils, N.; Camy, S.; Takache, H.; Ismail, A.; Pontalier, P.-Y. Supercritical carbon dioxide extraction and fractionation of lipids from freeze-dried microalgae Nannochloropsis oculata and Chlorella vulgaris. Algal Res. 2018, 34, 49–56. [Google Scholar] [CrossRef]
- Khorramdashti, M.S.; Giri, M.S.; Majidian, N. Extraction lipids from Chlorella vulgaris by supercritical CO2 for biodiesel production. S. Afr. J. Chem. Eng. 2021, 38, 121–131. [Google Scholar] [CrossRef]
- Wang, H.-M.; Pan, J.-L.; Chen, C.-Y.; Chiu, C.-C.; Yang, M.-H.; Chang, H.-W.; Chang, J.-S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process. Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Sovová, H.; Nobre, B.P.; Palavra, A. Modeling of the Kinetics of Supercritical Fluid Extraction of Lipids from Microalgae with Emphasis on Extract Desorption. Materials 2016, 9, 423. [Google Scholar] [CrossRef]
- Sovová, H. Rate of the vegetable oil extraction with supercritical CO2—I. Modelling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Sovová, H.; Kučera, J.; Jež, J. Rate of the vegetable oil extraction with supercritical CO2—II. Extraction of grape oil. Chem. Eng. Sci. 1994, 49, 415–420. [Google Scholar] [CrossRef]
- Soštarič, M.; Golob, J.; Bricelj, M.; Klinar, D.; Pivec, A. Studies on the growth of Chlorella vulgaris in culture media with different carbon sources. Chem. Biochem. Eng. Q. 2009, 23, 471–477. [Google Scholar]
- Liu, Y. Analysis on Extraction Behaviour of Lithium-ion Battery Electrolyte Solvents in Supercritical CO2 by Gas Chromatography. Int. J. Electrochem. Sci. 2016, 11, 7594–7604. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Engel, R.G. Isolation of chlorophyll and carotenoid pigments from spinach. In Introduction to Organic Laboratory Techniques: A Microscale Approach, 3rd ed.; Saunders College Publishing: New York, NY, USA, 1999. [Google Scholar]
- Vidussi, F.; Claustre, H.; Bustillos-Guzmàn, J.; Cailliau, C.; Marty, J.-C. Determination of chlorophylls and carotenoids of marine phytoplankton: Separation of chlorophyll a from divinylchlorophyll a and zeaxanthin from lutein. J. Plankton Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Pappa, G.D.; Louli, V.; Voutsas, E.; Magoulas, K. Experimental Design and Optimization of Recovering Bioactive Compounds from Chlorella vulgaris through Conventional Extraction. Molecules 2021, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Zaripheh, S.; Erdman, J.W. Factors That Influence the Bioavailablity of Xanthophylls. J. Nutr. 2002, 132, 531S–534S. [Google Scholar] [CrossRef]
- Pan, B.; Lei, J.; Zhang, L.; Guo, Y. Research on the physical properties of supercritical CO2 and the log evaluation of CO2-bearing volcanic reservoirs. J. Geophys. Eng. 2017, 14, 1052–1060. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Shi, X. Supercritical fluid extraction and determination of lutein in heterotrophically cultivated Chlorella pyrenoidosa. J. Food Process. Eng. 2007, 30, 174–185. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; LaRocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Yin, J.-Z.; Wang, A.-Q.; Wei, W.; Liu, Y.; Shi, W.-H. Analysis of the operation conditions for supercritical fluid extraction of seed oil. Sep. Purif. Technol. 2005, 43, 163–167. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Extraction of proanthocyanidins from grape marc by supercritical fluid extraction using CO2 as solvent and ethanol–water mixture as co-solvent. J. Supercrit. Fluids 2014, 87, 59–64. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Vidović, S.; Ilić, L.; Zeković, Z. Extraction kinetics and ANN simulation of supercritical fluid extraction of sage herbal dust. J. Supercrit. Fluids 2017, 130, 327–336. [Google Scholar] [CrossRef]
- del Valle, J.M.; Napolitano, P.; Fuentes, N. Estimation of Relevant Mass Transfer Parameters for the Extraction of Packed Substrate Beds Using Supercritical Fluids. Ind. Eng. Chem. Res. 2000, 39, 4720–4728. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M. Mass transfer modeling of Scenedesmus sp. lipids extracted by supercritical CO2. Biomass Bioenergy 2014, 70, 530–541. [Google Scholar] [CrossRef]
- Roy, B.C.; Goto, M.; Hirose, T.; Navaro, O.; Hortacsu, O. Extraction Rates of Oil from Tomato Seeds with Supercritical Carbon Dioxide. J. Chem. Eng. Jpn. 1994, 27, 768–772. [Google Scholar] [CrossRef]
- Kavoura, D.; Kyriakopoulou, K.; Papaefstathiou, G.; Spanidi, E.; Gardikis, K.; Louli, V.; Aligiannis, N.; Krokida, M.; Magoulas, K. Supercritical CO2 extraction of Salvia fruticosa. J. Supercrit. Fluids 2019, 146, 159–164. [Google Scholar] [CrossRef]
- FiorMarkets. Global Carotenoids Market Is Expected to Reach USD 3.59 Billion by 2025: Fior Markets. 2019. Available online: https://www.globenewswire.com/news-release/2019/10/15/1929461/0/en/Global-Carotenoids-Market-is-expected-to-reach-USD-3-59-billion-by-2025-Fior-Markets.html (accessed on 2 September 2022).
- Watson, S.B. Algal taste and odor. In Algae: Source to Treatment AWWA Manual of Water Supply Practices; AWWA Publishing: Denver, CO, USA, 2010; Volume 57, pp. 329–374. [Google Scholar]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Perez-Vega, S.; Salmeron, I.; Perez-Reyes, I.; Kwofie, E.; Ngadi, M. Influence of the Supercritical Fluid Extraction (SFE) on Food Bioactives. In Retention of Bioactives in Food Processing; Springer: Cham, Switzerland, 2022; pp. 309–340. [Google Scholar] [CrossRef]
- Yen, H.-W.; Yang, S.-C.; Chen, C.-H.; Jesisca; Chang, J.-S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2014, 184, 291–296. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sens. Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Augustynska, D.; Jemioła-Rzemińska, M.; Burda, K.; Strzałka, K. Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chem. Interact. 2015, 239, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Papamichail, I.; Louli, V.; Magoulas, K. Supercritical fluid extraction of celery seed oil. J. Supercrit. Fluids 2000, 18, 213–226. [Google Scholar] [CrossRef]
- Laina, K.M.; Eleni, P.N.; Tsitseli, K.G.; Krokida, M.K. Process Design for the Extraction of Bioactive Compounds from Several Mediterranean Medicinal Plants. Chem. Eng. Trans. 2021, 86, 1327–1332. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crop. Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Wright, S.W. Phytoplankton Pigments in Oceanography: Monographs on Oceanographic Methodology; UNESCO Publishing: Paris, France, 1997. [Google Scholar]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Reverchon, E.; Marrone, C. Supercritical extraction of clove bud essential oil: Isolation and mathematical modeling. Chem. Eng. Sci. 1997, 52, 3421–3428. [Google Scholar] [CrossRef]









| Run | T (°C) | P (bar) | Flow (g/min) | Time (h) | Yield (% w/w) | IC50 (mgextr/ mgDPPH) | TPC (mgGA/gextr) | CHL (mg/gextr) | Sel. CAR (mg/gextr) | CAR (mg/gextr) |
|---|---|---|---|---|---|---|---|---|---|---|
| SFE-1 | 40 | 180 | 30 | 4.44 | 1.54 | 61.36 | 12.04 | 22.71 | 7.48 | 16.52 |
| SFE-2 | 40 | 110 | 20 | 6.67 | 1.25 | 121.64 | 7.27 | 1.90 | 1.76 | 3.91 |
| SFE-3 | 40 | 110 | 40 | 3.33 | 1.77 | 97.97 | 14.13 | 12.08 | 2.05 | 3.15 |
| SFE-4 | 40 | 250 | 20 | 6.67 | 1.27 | 46.48 | 7.76 | 28.86 | 8.44 | 20.04 |
| SFE-5 | 40 | 250 | 40 | 3.33 | 1.83 | 54.27 | 20.76 | 31.26 | 10.69 | 22.51 |
| SFE-6 | 50 | 110 | 30 | 4.44 | 1.90 | 204.83 | 9.17 | 1.33 | 0.65 | 1.59 |
| SFE-7 | 50 | 180 | 20 | 6.67 | 1.55 | 105.36 | 9.87 | 4.71 | 2.08 | 5.67 |
| SFE-8 | 50 | 180 | 30 | 4.44 | 2.11 | 80.43 | 12.50 | 12.08 | 4.55 | 8.30 |
| SFE-9 | 50 | 180 | 30 | 4.44 | 1.96 | 74.76 | 10.11 | 11.37 | 3.77 | 7.43 |
| SFE-10 | 50 | 180 | 30 | 4.44 | 1.99 | 68.25 | 14.26 | 14.25 | 385 | 10.03 |
| SFE-11 | 50 | 180 | 30 | 4.44 | 2.10 | 75.01 | 9.01 | 15.16 | 3.98 | 10.96 |
| SFE-12 | 50 | 180 | 40 | 3.33 | 2.19 | 68.03 | 7.81 | 17.79 | 5.91 | 14.41 |
| SFE-13 | 50 | 250 | 30 | 4.44 | 2.50 | 54.38 | 11.51 | 31.23 | 9.68 | 20.81 |
| SFE-14 | 60 | 110 | 20 | 6.67 | 2.45 | 226.12 | 4.06 | 1.66 | 0.17 | 0.66 |
| SFE-15 | 60 | 110 | 40 | 3.33 | 2.42 | 317.03 | 5.57 | 2.44 | 0.15 | 0.87 |
| SFE-16 | 60 | 250 | 20 | 6.67 | 3.38 | 47.88 | 21.38 | 25.18 | 5.82 | 16.59 |
| SFE-17 | 60 | 250 | 40 | 3.33 | 3.37 | 44.35 | 18.29 | 32.55 | 10.00 | 21.14 |
| SFE-18 | 60 | 180 | 30 | 4.44 | 3.05 | 67.30 | 7.30 | 9.89 | 3.96 | 11.40 |
| SD * (%) | ±3.22 | ±5.78 | ±17.84 | ±11.68 | ±7.54 | ±15.14 | ||||
| Yield | IC50 | Sel. CAR. | CAR | ||||
|---|---|---|---|---|---|---|---|
| Source | p-Value | Source | p-Value | Source | p-Value | Source | p-Value |
| Model | <0.0001 | Model | <0.0001 | Model | <0.0001 | Model | <0.0001 |
| T | <0.0001 | P | 0.005 | P | 0.0001 | T | 0.0236 |
| P | 0.0003 | F | 0.0426 | F | 0.0019 | P | 0.0013 |
| F | 0.0005 | TP | 0.0005 | P2 | 0.0004 | F | 0.0084 |
| TP | 0.0001 | T2 | 0.0479 | F2 | 0.0276 | TP | 0.0007 |
| TF | 0.003 | P2 | 0.0018 | T2F | 0.0104 | P2 | 0.0002 |
| T2 | 0.004 | T2F | 0.0432 | TP2 | 0.0208 | T2F | 0.0259 |
| P2 | 0.05 | ||||||
| F2 | 0.01 | ||||||
| LOF | 0.296 | 0.1025 | 0.1080 | 0.4358 | |||
| R2 | 0.987 | 0.9717 | 0.9941 | 0.9832 | |||
| Adj-R2 | 0.976 | 0.9466 | 0.9858 | 0.9683 | |||
| Pred-R2 | 0.944 | 0.7934 | 0.8934 | 0.9372 | |||
| Adeq Prec | 32.11 | 20.52 | 35.97 | 25.12 | |||
| P (bar) | T (°C) | F (g/min) | yr | x0 | xk | 102 (s−1) | 104 (s−1) | AAD * (%) |
|---|---|---|---|---|---|---|---|---|
| 250 | 40 | 40 | 0.0006 | 0.018 | 0.0132 | 1.18 | 3.02 | 3.29 |
| 110 | 60 | 40 | 0.0012 | 0.025 | 0.0167 | 2.38 | 3.83 | 2.04 |
| 250 | 60 | 20 | 0.0032 | 0.036 | 0.0175 | 1.18 | 1.58 | 3.03 |
| 250 | 60 | 40 | 0.0032 | 0.036 | 0.0175 | 1.18 | 2.86 | 1.66 |
| Parameter | SLE [34] | SFE |
|---|---|---|
| solvent | EtOH 90% v/v | CO2 |
| solvent-to-biomass (kg/kgbiom) | 30 | 100 |
| stirring (rpm) | 500 | n/a * |
| T (°C) | 30 | 60 |
| P (bar) | 1 | 250 |
| F (g/min) | n/a * | 40 |
| Duration (h) | 24 | 3.3 |
| Primary Composition | % * |
|---|---|
| Lipid | 22.17 ± 0.46 |
| Carbohydrate | 33.84 ± 1.33 |
| Protein | 44.48 ± 0.77 |
| Ash | 5.63 ± 0.06 |
| Moisture | 2.32 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Supercritical CO2 Extraction of High-Added Value Compounds from Chlorella vulgaris: Experimental Design, Modelling and Optimization. Molecules 2022, 27, 5884. https://doi.org/10.3390/molecules27185884
Georgiopoulou I, Tzima S, Louli V, Magoulas K. Supercritical CO2 Extraction of High-Added Value Compounds from Chlorella vulgaris: Experimental Design, Modelling and Optimization. Molecules. 2022; 27(18):5884. https://doi.org/10.3390/molecules27185884
Chicago/Turabian StyleGeorgiopoulou, Ioulia, Soultana Tzima, Vasiliki Louli, and Kostis Magoulas. 2022. "Supercritical CO2 Extraction of High-Added Value Compounds from Chlorella vulgaris: Experimental Design, Modelling and Optimization" Molecules 27, no. 18: 5884. https://doi.org/10.3390/molecules27185884
APA StyleGeorgiopoulou, I., Tzima, S., Louli, V., & Magoulas, K. (2022). Supercritical CO2 Extraction of High-Added Value Compounds from Chlorella vulgaris: Experimental Design, Modelling and Optimization. Molecules, 27(18), 5884. https://doi.org/10.3390/molecules27185884