Design, Synthesis and Bioactivity of Novel Low Bee-Toxicity Compounds Based on Flupyrimin
Abstract
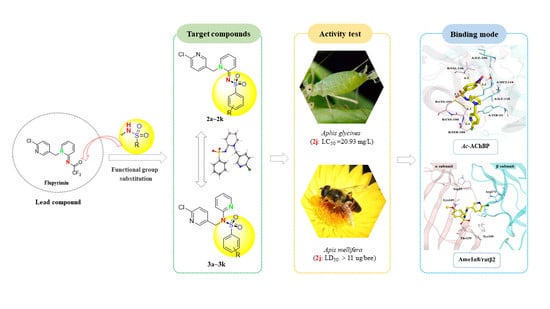
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Target Compounds
2.2. Structural Analysis
2.3. Insecticidal Activity against Adult Aphis glycines by the Target Compounds
2.4. Oral and Contact Toxicity to Apis mellifera
2.5. Molecular Docking of Compounds with Nicotinic Acetylcholine Receptors
2.5.1. Molecular Docking of Compounds with Insect AchBP
2.5.2. Molecular Docking of Compounds with Honeybee nAChR Subunits AmeIα8/ratβ2
2.6. DFT Calculation of Compounds 2j and FLP
3. Materials and Methods
3.1. Chemicals and Target Compounds
3.1.1. Preparation of N-(pyridin-2-yl) Substituted Benzene Sulfonamide
3.1.2. Preparation of (E)-N-(1-((6-chloropyridin-3-yl)methyl)pyridin-2(1H)-ylidene)benzenesulfonamide (2a) and N-((6-chloropyridin-3-yl)methyl)-N-(pyridin-2-yl)benzenesulfonamide (3a)
3.1.3. Chemical Properties of the Compounds
3.2. Insecticidal Activity Test
3.3. Oral and Contact Toxicity to Apis mellifera
3.4. Molecular Docking
3.5. Density Functional Theory (DFT) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dedryver, C.A.; le Ralec, A.; Fabre, F. The Conflicting Relationships between Aphids and Men: A Review of Aphid Damage and Control Strategies. Comptes Rendus Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Giordanengo, P.; Brunissen, L.; Rusterucci, C.; Vincent, C.; van Bel, A.; Dinant, S.; Girousse, C.; Faucher, M.; Bonnemain, J.L. Compatible Plant-Aphid Interactions: How Aphids Manipulate Plant Responses. Comptes Rendus Biol. 2010, 333, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as Transport Devices for Plant Viruses. Comptes Rendus Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]
- Zeni, V.; Baliota, G.V.; Benelli, G.; Canale, A.; Athanassiou, C.G. Diatomaceous Earth for Arthropod Pest Control: Back to the Future. Molecules 2021, 26, 7487. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Bielza, P.; Denholm, I.; Gorman, K. Age-Specific Expression of Resistance to a Neonicotinoid Insecticide in the Whitefly Bemisia Tabaci. Pest Manag. Sci. 2008, 64, 1106–1110. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, J.; Xu, Z.; Xu, X.; Shao, X.; Li, Z. Synthesis and Biological Activity Evaluation of Novel β-Substituted Nitromethylene Neonicotinoid Analogues. Molecules 2012, 17, 10014–10025. [Google Scholar] [CrossRef]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B.; Buckingham, S.D. Neonicotinoids: Insecticides Acting on Insect Nicotinic Acetylcholine Receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal Structure of an ACh-Binding Protein Reveals the Ligand-Binding Domain of Nicotinic Receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef]
- Celie, P.H.N.; van Rossum-Fikkert, S.E.; van Dijk, W.J.; Brejc, K.; Smit, A.B.; Sixma, T.K. Nicotine and Carbamylcholine Binding to Nicotinic Acetylcholine Receptors as Studied in AChBP Crystal Structures. Neuron 2004, 41, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Ihara, M.; Okajima, T.; Yamashita, A.; Oda, T.; Hirata, K.; Nishiwaki, H.; Morimoto, T.; Akamatsu, M.; Ashikawa, Y.; Kuroda, S.; et al. Crystal Structures of Lymnaea Stagnalis AChBP in Complex with Neonicotinoid Insecticides Imidacloprid and Clothianidin. Invertebr. Neurosci. 2008, 8, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Talley, T.T.; Harel, M.; Hibbs, R.E.; Radić, Z.; Tomizawa, M.; Casida, J.E.; Taylor, P. Atomic Interactions of Neonicotinoid Agonists with AChBP: Molecular Recognition of the Distinctive Electronegative Pharmacophore. Proc. Natl. Acad. Sci. USA 2008, 105, 7606–7611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, R.; Zhang, D.; Wu, R.; Liu, T.; Wu, Z.; Song, B. New Synthetic Method and Insecticidal Activities of Novel Imidazopyridine Mesoionic Derivatives Containing an Ester Group. J. Agric. Food Chem. 2022, 70, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Liu, D.; Liu, Z.; Shi, J.; He, W.; Qi, P.; Chen, J.; Song, B. Design, Synthesis, and Insecticidal Activity Evaluation of Novel 4-(N, N-Diarylmethylamines)Furan-2(5H)-One Derivatives as Potential Acetylcholine Receptor Insecticides. Pest Manag. Sci. 2019, 75, 427–437. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A Worldwide Survey of Neonicotinoids in Honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Dong, F.; Duan, H.; Shao, X.; Chen, X.; Yang, T.; Wang, G.; Zheng, Y. Ecological Toxicity Reduction of Dinotefuran to Honeybee: New Perspective from an Enantiomeric Level. Environ. Int. 2019, 130, 104854. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Z.; Zhu, K.; Li, H.; Qin, Z.; Duan, H. Computational Insight on the Binding and Selectivity of Target-Subunit-Dependent for Neonicotinoid Insecticides. J. Mol. Graph. Model. 2020, 98, 107586. [Google Scholar] [CrossRef]
- Holyoke, C.W.; Zhang, W.; Pahutski, T.F.; Lahm, G.P.; Tong, M.H.T.; Cordova, D.; Schroeder, M.E.; Benner, E.A.; Rauh, J.J.; Dietrich, R.F.; et al. Triflumezopyrim: Discovery and Optimization of a Mesoionic Insecticide for Rice. ACS Symp. Ser. 2015, 1204, 365–378. [Google Scholar] [CrossRef]
- Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K.A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; et al. Flupyrimin: A Novel Insecticide Acting at the Nicotinic Acetylcholine Receptors. J. Agric. Food Chem. 2017, 65, 7865–7873. [Google Scholar] [CrossRef]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A Brief Profile of a New Butenolide Insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.B.; Siebert, M.W.; Wang, N.X.; Loso, M.R.; Sparks, T.C. Sulfoxaflor—A Sulfoximine Insecticide: Review and Analysis of Mode of Action, Resistance and Cross-Resistance. Pestic. Biochem. Physiol. 2021, 178, 104924. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, D.; Gan, X.; Zhang, J.; Liu, Z.; Yi, C.; Song, B. Synthesis and Biological Activity of Novel 1,3,4-Thiadiazolo[3,2-a]Pyrimidinone Mesoionic Derivatives. Chin. J. Org. Chem. 2019, 39, 2287–2294. [Google Scholar] [CrossRef]
- Harmata, M.; Zheng, P.; Huang, C.; Gomes, M.G.; Ying, W.; Ranyanil, K.; Balan, G.; Calkins, N.L.; Chem, J.A.P.A. Expedient Synthesis of Sulfinamides from Sulfonyl Chlorides Sulfinamides Were Synthesized from Sulfonyl Chlorides Using a Procedure Involving in Situ Reduction of Sulfonyl Chlorides. The Reaction Is Broad in Scope and Easy to Perform. Sulfinamides, Esp. J. Org. Chem. 2007, 72, 683–685. [Google Scholar] [CrossRef]
- Cho, S.Y.; Fox, E.; McCully, C.; Bauch, J.; Marsh, K.; Balis, F.M. Plasma and Cerebrospinal Fluid Pharmacokinetics of Intravenously Administered ABT-751 in Non-Human Primates. Cancer Chemother. Pharmacol. 2007, 60, 563–567. [Google Scholar] [CrossRef]
- Kumar Parai, M.; Panda, G.; Srivastava, K.; Kumar Puri, S. Design, Synthesis and Antimalarial Activity of Benzene and Isoquinoline Sulfonamide Derivatives. Bioorganic Med. Chem. Lett. 2008, 18, 776–781. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Wei, J.; Zhu, F.; Gang, F.; Wei, S.; Zhao, Y.; Zhang, J.; Wu, W. Synthesis and Insecticidal Activity In Vitro and Vivo of Novel Benzenesulfonyl Derivatives Based on Potent Target Subunit H of V-ATPase. Bioorganic Med. Chem. Lett. 2018, 28, 3164–3167. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Zhang, J.; Lu, X.; Li, X.; Jiang, Z.; Song, D.; Duan, H.; Yang, X. Screening and Optimization of Novel Low Bee-Toxicity Phenylacetohydrazone Compounds Based on Insect NAChR Selectivity. Chin. J. Org. Chem. 2021, 41, 2774–2787. [Google Scholar] [CrossRef]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The Worldwide Importance of Honey Bees as Pollinators in Natural Habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 2140. [Google Scholar] [CrossRef]
- WSDA. Pollinator Protection Requirements for Section 18 Emergency Exemptions and Section 24(c) Special Local Need Registration in Washington State; WSDA: Washington, DC, USA, 2010; p. 9.
- Zhang, X.; Xu, H.; Su, H.; Yang, X.; Sun, T.; Lu, X.; Shi, F.; Duan, H.; Liu, X.; Ling, Y. Design, Synthesis, and Biological Activity of Novel Fungicides Containing a 1,2,3,4-Tetrahydroquinoline Scaffold and Acting as Laccase Inhibitors. J. Agric. Food Chem. 2022, 70, 1776–1787. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, A.; Ren, Y.; Zhou, C.; Cheng, S.; Xiang, J.; Liu, X.; Liu, M.; Huang, M.; Liu, Z. Synthesis and Biological Activity of the Novel Insecticide: Flupyrimin. FINE Chem. Intermed. 2018, 48, 12–14. [Google Scholar]
- El-Zemity, S.R.; Badawy, M.E.; Khattab, M.M.; Marei, A.E.-S. Structure and Acaridical Activity Relationship of Some Sulfonamide Derivatves against the Two-Spotted Spider Mite, Tetranychus Urticae (Koch). Int. J. Agric. Biol. 2006, 8, 661–665. [Google Scholar]
- Abdullah, I.; Gary, S.R.; Marla, S. Field Trial of Honey Bee Colonies Bred for Mechanisms of Resistance against Varroa Destructor. Apidologie 2007, 38, 67–76. [Google Scholar] [CrossRef]
- Duke, O.S.; Powles, B.S. Glyphosate: A Once-in-a-Century Herbicide. Pest Manag. Sci. 2008, 63, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Oruc, H.H.; Hranitz, J.M.; Sorucu, A.; Duell, M.; Cakmak, I.; Aydin, L.; Orman, A. Determination of Acute Oral Toxicity of Flumethrin in Honey Bees. J. Econ. Entomol. 2012, 105, 1890–1894. [Google Scholar] [CrossRef]










| Entry | Base | Solvent | 2a Yield % | 3a Yield % |
|---|---|---|---|---|
| 1 | K2CO3 | MeOH | 29.41 | 14.05 |
| 2 | K2CO3 | THF | 32.68 | 16.99 |
| 3 | K2CO3 | MeCN | 39.54 | 20.26 |
| 4 | K2CO3 | DMSO | 49.35 | 11.44 |
| 5 | K2CO3 | DMF | 68.95 | 15.36 |
| 6 | Cs2CO3 | DMF | 65.95 | 16.01 |
| 7 | Et3N | DMF | 26.14 | 16.01 |
| 8 | DIPEA | DMF | 67.97 | 15.03 |
| 9 | NaH | DMF | 64.30 | 10.30 |
| 10 | NaOH | DMF | 65.36 | 0 |
| Compd. | R | Mortality Rate ± St. D a/% | Compd. | R | Mortality Rate ± St. D/% |
|---|---|---|---|---|---|
| 2a | H | 13.96 ± 1.82 | 3a | H | 46.51 ± 7.91 |
| 2b | 2-CH3 | 18.61 ± 4.80 | 3b | 2-CH3 | 55.81 ± 3.63 |
| 2c | 3-CH3 | 23.26 ± 3.14 | 3c | 3-CH3 | 72.09 ± 3.14 |
| 2d | 4-CH3 | 39.54 ± 3.63 | 3d | 4-CH3 | 32.56 ± 4.80 |
| 2e | 2-Cl | 37.21 ± 11.33 | 3e | 2-Cl | 51.17 ± 3.14 |
| 2f | 3-Cl | 60.47 ± 6.54 | 3f | 3-Cl | 18.61 ± 3.63 |
| 2g | 4-Cl | 76.75 ± 4.80 | 3g | 4-Cl | 30.24 ± 6.29 |
| 2h | 3-Br | 68.15 ± 11.65 | 3h | 3-Br | 17.65 ± 5.77 |
| 2i | 4-OCH3 | 49.02 ± 5.44 | 3i | 4-OCH3 | 9.80 ± 2.72 |
| 2j | 4-NO2 | 94.12 ± 2.89 | 3j | 4-NO2 | 17.65 ± 9.43 |
| 2k | 2,4-2Cl | 57.50 ± 6.35 | 3k | 2,4-2Cl | 13.73 ± 2.72 |
| PYM | 81.62 ± 4.97 | FLP | 100 |
| Compd. | Y = ax + b | LC50 (mg/L) | 95% CI a | LC90 (mg/L) | r | χ2 |
|---|---|---|---|---|---|---|
| 2g | y = 1.25x − 2.26 | 63.74 | 51.85–80.51 | 666.88 | 0.96 | 5.68 |
| 2j | y = 1.85x − 2.44 | 20.93 | 16.18–26.26 | 336.51 | 0.98 | 2.26 |
| 3c | y = 1.85x − 3.71 | 102.38 | 87.35–123.14 | 463.35 | 0.97 | 6.31 |
| PYM | y = 1.92x − 1.90 | 9.98 | 4.37–21.40 | 47.479 | 0.98 | 5.00 |
| FLP | y = 1.99x + 1.47 | 0.19 | 0.12–0.29 | 0.934 | 0.95 | 4.47 |
| Compd. | Mortality Rate % (Dose) | Bee-Toxicity b | |
|---|---|---|---|
| Oral Toxicity | Contact Toxicity | ||
| 2j | 3.33 (12 μg/bee) | 13.33 (13.34 μg/bee) | Low |
| FLP | 6.67 (60 μg/bee) | 0 (30 μg/bee) | Low |
| IMIa | 2.82 × 10−2 | 5.78 × 10−2 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Xu, H.; Zhang, X.; Sun, T.; Lin, Y.; Zhang, Y.; Li, H.; Li, X.; Yang, X.; Duan, H.; et al. Design, Synthesis and Bioactivity of Novel Low Bee-Toxicity Compounds Based on Flupyrimin. Molecules 2022, 27, 6133. https://doi.org/10.3390/molecules27186133
Lu X, Xu H, Zhang X, Sun T, Lin Y, Zhang Y, Li H, Li X, Yang X, Duan H, et al. Design, Synthesis and Bioactivity of Novel Low Bee-Toxicity Compounds Based on Flupyrimin. Molecules. 2022; 27(18):6133. https://doi.org/10.3390/molecules27186133
Chicago/Turabian StyleLu, Xingxing, Huan Xu, Xiaoming Zhang, Tengda Sun, Yufan Lin, Yongheng Zhang, Honghong Li, Xuesheng Li, Xinling Yang, Hongxia Duan, and et al. 2022. "Design, Synthesis and Bioactivity of Novel Low Bee-Toxicity Compounds Based on Flupyrimin" Molecules 27, no. 18: 6133. https://doi.org/10.3390/molecules27186133
APA StyleLu, X., Xu, H., Zhang, X., Sun, T., Lin, Y., Zhang, Y., Li, H., Li, X., Yang, X., Duan, H., & Ling, Y. (2022). Design, Synthesis and Bioactivity of Novel Low Bee-Toxicity Compounds Based on Flupyrimin. Molecules, 27(18), 6133. https://doi.org/10.3390/molecules27186133