β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity
Abstract
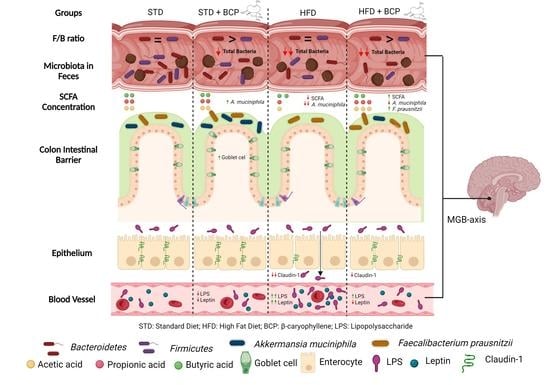
:1. Introduction
2. Results
2.1. β-Caryophyllene Increases the Number of Goblet Cells and the Thickness of Mucus in the Colon of Mice on a Standard Diet
2.2. β-Caryophyllene Restores the Claudin-1 Protein Expression in Mice with Diet-Induced Obesity
2.3. β-Caryophyllene Decreases the Metabolic Endotoxemia Levels in Mice with Diet-Induced Obesity
2.4. β-Caryophyllene Selectively Modulates the Microbial Abundance in Feces
2.5. Influence of β-Caryophyllene on the Production of Metabolites of the Microbiota (Short Chain Fatty Acids) in the Ascending Colon and Feces
2.6. β-Caryophyllene Administration Decreases the Serum Leptin Levels in Mice
3. Discussion
4. Materials and Methods
4.1. β-Caryophyllene
4.2. Diet
4.3. Animals
4.4. Experimental Design
4.5. Goblet Cell Count
4.6. Immunohistochemistry
4.7. LPS Quantification
4.8. Microbial Abundance (qPCR)
4.9. Short-Chain Fatty Acids (Gas Chromatography)
4.10. Serum Leptin Concentration (ELISA)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Calculation of the Sample Size
- n = sample size
- Z = confidence level considering the p to be used = 1.96
- σ = variation obtained in the measured parameter = 5.6
- δ = desired variation of the parameter = 3
Appendix A.2. LPS Quantification
Appendix A.3. Short-Chain Fatty Acids (Gas Chromatography)
Appendix B
Appendix B.1. Effect of β-Caryophyllene on Body Weight Gain


Appendix B.2. Effect of β-Caryophyllene on the Goblet Cell Numbers and the Mucus Layer


Appendix B.3. Effect of β-Caryophyllene on the Claudin-1 Protein Expression in the Colon

Appendix B.4. Effect of β-Caryophyllene on the Serum LPS Concentration (Endotoxemia)
| Sample | Control/Spike | ||||||
|---|---|---|---|---|---|---|---|
| ID | Dilution | EU/mL | % C.V. | EU/mL | % C.V. | % Recovery | |
| Non-Fasting | STD | 1:100 | 62.1 | 1.2 | 1.73 | 2.5 | 199 |
| STD + BCP | 1:100 | 63.8 | 11.6 | 1.59 | 7.4 | 183 | |
| HFD | 1:1000 | 124 | 0.2 | 1.61 | 6.2 | 185 | |
| HDF + BCP | 1:100 | 56.2 | 2.4 | 0.678 | 5.6 | 78 | |
| Fasting | STD | 1:100 | 12.8 | 2.2 | 0.690 | 2.7 | 79 |
| STD + BCP | 1:100 | 10.8 | 16.2 | 0.867 | 3.3 | 100 | |
| HFD | 1:1000 | 120 | 0.7 | 1.19 | 10.4 | 137 | |
| HDF + BCP | 1:100 | 46.5 | 18 | 1.46 | 5.3 | 168 | |
Appendix B.5. β-Caryophyllene on the Microbial Abundance in Feces

Appendix B.6. Concentration of the Short Chain Fatty Acids in the Colonic Content and Feces
| STD | STD + BCP | HFD | HFD+ BCP | |
|---|---|---|---|---|
| Acetic acid | 0.295 ± 0.093 | 0.215 ± 0.051 * | 0.392 ± 0174 * | 0.302 ± 0.093 |
| Propionic acid | 0.229 ± 0.054 | 0.203 ± 0.027 | 0.221 ± 0.055 | 0.245 ± 0.073 |
| Butyric acid | 0.264 ± 0.090 | 0.166 ± 0.061 | 0.169 ± 0.031 | 0.291 ± 0.179 |
| Total fatty acids | 0.788 ± 0.179 | 0.584 ± 0.129 | 0.782 ± 0.224 | 0.840 ± 0.305 |

| STD | STD + BCP | HFD | HFD+ BCP | |
|---|---|---|---|---|
| Acetic acid | 0.144 ± 0.713 | 0.150 ± 0.064 | 0.127 ± 0.044 * | 0.215 ± 0.073 * |
| Propionic acid | 0.199 ± 0.043 # | 0.193 ± 0.047 | 0.101 ± 0.095 #** | 0.219 ± 0.085 ** |
| Butyric acid | 0.045 ± 0.018 | 0.041 ± 0.014 | 0.031 ± 0.008 | 0.043 ± 0.016 |
| Total fatty acids | 0.388 ± 0.125 | 0.385 ± 0.102 | 0.261 ± 0.136 ** | 0.478 ± 0.166 ** |

Appendix B.7. Concentration of Serum Leptin Levels in Mice
| STD | STD + BCP | HFD | HFD+ BCP | |
|---|---|---|---|---|
| Leptin | 142.2 ± 78.30 | 89.6 ± 42.48 | 287.5 ± 122.5 | 133.4 ± 43.14 |
References
- Perez-Campos, E.; Mayoral, L.P.-C.; Andrade, G.M.; Mayoral, E.P.-C.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.R.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.P.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Franco-Arroyo, N.N.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.M.; Roldán, E.; Márquez-Aguirre, A.L.; Zepeda-Nuño, J.S.; Velázquez-Juárez, G.; Fafutis-Morris, M.; López-Roa, R.I. β-Caryophyllene, a Dietary Cannabinoid, Protects against Metabolic and Immune Dysregulation in a Diet-Induced Obesity Mice Model. J. Med. Food 2022. published online ahead of print. [Google Scholar] [CrossRef]
- Nakadate, K.; Hirakawa, T.; Tanaka-Nakadate, S. Small intestine barrier function failure induces systemic inflammation in monosodium glutamate-induced chronically obese mice. Appl. Physiol. Nutr. Metab. 2019, 44, 587–594. [Google Scholar] [CrossRef]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; Roa, R.I.L.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice. Nutrients 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Winer, D.A.; Luck, H.; Tsai, S.; Winer, S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016, 23, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Rah, B.; Bastola, D.; Dhawan, P.; Singh, A.B. Obesity-induces Organ and Tissue Specific Tight Junction Restructuring and Barrier Deregulation by Claudin Switching. Sci. Rep. 2017, 7, 5125. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Mahallei, M.; Pormohammad, A.; Varshochi, M.; Ganbarov, K.; Zeinalzadeh, E.; Yousefi, B.; Bastami, M.; Tanomand, A.; Mahmood, S.S.; et al. Microbial balance in the intestinal microbiota and its association with diabetes, obesity and allergic disease. Microb. Pathog. 2019, 127, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes. Molecules 2020, 25, 691. [Google Scholar] [CrossRef]
- Verty, A.N.A.; Stefanidis, A.; McAinch, A.J.; Hryciw, D.H.; Oldfield, B. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS ONE 2015, 10, e0140592. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. The endocannabinoid system in inflammatory bowel diseases: From pathophysiology to therapeutic opportunity. Trends Mol. Med. 2012, 18, 615–625. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Birchenough, G.M.; Pradhan, M.; Nyström, E.E.; Henricsson, M.; Hansson, G.C.; Bäckhed, F. Obesity-associated microbiota contributes to mucus layer defects in genetically obese mice. J. Biol. Chem. 2020, 295, 15712. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Shen, L.; Zhang, R.; Yang, L.; Li, M.; Li, K. The Anti-Inflammatory Effect and Intestinal Barrier Protection of HU210 Differentially Depend on TLR4 Signaling in Dextran Sulfate Sodium-Induced Murine Colitis. Dig Dis Sci. 2017, 62, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2015, 12, 133–143. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.E2. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. -Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Upadhyay, V.; Turnbaugh, J.A.; Ly, K.; Turnbaugh, P.J. Meta-Analysis Reveals Reproducible Gut Microbiome Alterations in Response to a High-Fat Diet. Cell Host Microbe 2019, 26, 265–272.e4. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-J.; Jwa, S.-K. Efficacy of β-caryophyllene for periodontal disease related factors. Arch. Oral Biol. 2019, 100, 113–118. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front. Cell. Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Koonen, D.; Hofker, M.; Fu, J. Gut microbiome and lipid metabolism: From associations to mechanisms. Curr. Opin. Lipidol. 2016, 27, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Crouch, L.I.; Liberato, M.V.; Urbanowicz, P.A.; Baslé, A.; Lamb, C.A.; Stewart, C.J.; Cooke, K.; Doona, M.; Needham, S.; Brady, R.R.; et al. Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nat. Commun. 2020, 11, 591568. [Google Scholar] [CrossRef]
- Anastasovska, J.; Arora, T.; Canon, G.J.S.; Parkinson, J.R.; Tuohy, K.; Gibson, G.R.; Nadkarni, N.A.; So, P.-W.; Goldstone, A.; Thomas, E.; et al. Fermentable Carbohydrate Alters Hypothalamic Neuronal Activity and Protects against the Obesogenic Environment. Obesity 2012, 20, 1016–1023. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [Green Version]
- den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Yan, H.; Wang, Q.; Wang, H.; et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 2021, 87–88, 111198. [Google Scholar] [CrossRef]
- Pathak, M.P.; Patowary, P.; Goyary, D.; Das, A.; Chattopadhyay, P. β-caryophyllene ameliorated obesity-associated airway hyperresponsiveness through some non-conventional targets. Phytomedicine 2021, 89, 153610. [Google Scholar] [CrossRef]
- Godínez-Méndez, L.A.; Gurrola-Díaz, C.M.; Zepeda-Nuño, J.S.; Vega-Magaña, N.; Lopez-Roa, R.I.; Íñiguez-Gutiérrez, L.; García-López, P.M.; Fafutis-Morris, M.; Delgado-Rizo, V. In Vivo Healthy Benefits of Galacto-Oligosaccharides from Lupinus albus (LA-GOS) in Butyrate Production through Intestinal Microbiota. Biomolecules 2021, 11, 1658. [Google Scholar] [CrossRef]
- Ribeiro, W.; Vinolo, M.; Calixto, L.; Ferreira, C. Use of Gas Chromatography to Quantify Short Chain Fatty Acids in the Serum, Colonic Luminal Content and Feces of Mice. Bio-Protocol 2018, 8, e3089. [Google Scholar] [CrossRef] [Green Version]







| Gene | Forward | Reverse | Product | Temperature |
|---|---|---|---|---|
| 16srDNA | AGTTTGATCCTGGCTCAG | GWATTACCGCGGCKGCTG | 516 | 62 °C |
| Bacteroidetes | GGARCATGTGGTTTAATTCGATGAT | AGCTGACGACAACCATGCAG | 126 | 63 °C |
| Firmicutes | GGAGYATGTGGTTTAATTCGAAGCA | AGCTGACGACAACCATGCAC | 129 | 60 °C |
| A. muciniphila | CAGCACGTGAAGGTGGGGAC | CCTTGCGGTTGGCTTCAGAT | 329 | 60 °C |
| F. prausnitzii | GATGGCCTCGCGTCCGATTAG | CCGAAGACCTTCTTCCTC | 129 | 57 °C |
| R. torques | AATCTTCGGAGGAAGAGGACA | ACACTACACCATGCGGTCCT | 137 | 56 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Mejía, U.U.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Zepeda-Nuño, J.S.; Velázquez-Juárez, G.; Delgado-Rizo, V.; García-Iglesias, T.; Camacho-Padilla, L.G.; Varela-Navarro, E.; et al. β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity. Molecules 2022, 27, 6156. https://doi.org/10.3390/molecules27196156
Rodríguez-Mejía UU, Viveros-Paredes JM, Zepeda-Morales ASM, Carrera-Quintanar L, Zepeda-Nuño JS, Velázquez-Juárez G, Delgado-Rizo V, García-Iglesias T, Camacho-Padilla LG, Varela-Navarro E, et al. β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity. Molecules. 2022; 27(19):6156. https://doi.org/10.3390/molecules27196156
Chicago/Turabian StyleRodríguez-Mejía, Uriel Ulises, Juan Manuel Viveros-Paredes, Adelaida Sara Minia Zepeda-Morales, Lucrecia Carrera-Quintanar, José Sergio Zepeda-Nuño, Gilberto Velázquez-Juárez, Vidal Delgado-Rizo, Trinidad García-Iglesias, Luisa Guadalupe Camacho-Padilla, Elizabeth Varela-Navarro, and et al. 2022. "β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity" Molecules 27, no. 19: 6156. https://doi.org/10.3390/molecules27196156
APA StyleRodríguez-Mejía, U. U., Viveros-Paredes, J. M., Zepeda-Morales, A. S. M., Carrera-Quintanar, L., Zepeda-Nuño, J. S., Velázquez-Juárez, G., Delgado-Rizo, V., García-Iglesias, T., Camacho-Padilla, L. G., Varela-Navarro, E., Anguiano-Sevilla, L. A., Franco-Torres, E. M., & López-Roa, R. I. (2022). β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity. Molecules, 27(19), 6156. https://doi.org/10.3390/molecules27196156