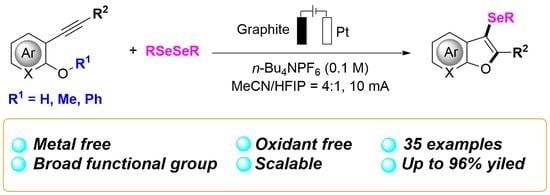
Direct Electrooxidative Selenylation/Cyclization of Alkynes: Access to Functionalized Benzo[b]furans
Abstract
:1. Introduction
2. Results and Dissections
2.1. Optimization of Reaction Conditions
2.2. Substrate Scope
2.3. Scaled-Up Synthesis and Transformation
2.4. Control Experiments and Plausible Mechanism
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Materials
4.3. Procedure for the Electrosynthesis of Compounds 3
4.4. Procedure for the Electrosynthesis of Compounds 4
4.5. Procedure for the Scaled-Up Synthesis of Compound 3a and 3t
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Zhong, Y.-F.; Mo, Z.-Y.; Wu, S.-H.; Xu, Y.-L.; Tang, H.-T.; Pan, Y.-M. Synthesis of Seleno Oxindoles via Electrochemical Cyclization of N-arylacrylamides with Diorganyl Diselenides. Adv. Synth. Catal. 2021, 363, 208–214. [Google Scholar] [CrossRef]
- Wang, L.-W.; Feng, Y.-F.; Lin, H.-M.; Tang, H.-T.; Pan, Y.-M. Electrochemically Enabled Selenium Catalytic Synthesis of 2,1-Benzoxazoles from o-Nitrophenylacetylenes. J. Org. Chem. 2021, 86, 16121–16127. [Google Scholar] [CrossRef] [PubMed]
- Manjare, S.T.; Kim, Y.; Churchill, D.G. Selenium- and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef]
- Selvakumar, K.; Singh, H.B. Adaptive Responses of Sterically Confined Intramolecular Chalcogen Bonds. Chem. Sci. 2018, 9, 7027–7042. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Han, C.; Zuo, D.; Zhai, M.; Li, Z.; Zhang, Q.; Zhai, Y.; Jiang, X.; Bao, K.; Wu, Y.; et al. Synthesis and evaluation of benzimidazole carbamates bearing indole moieties for antiproliferative and antitubulin activities. Eur. J. Med. Chem. 2014, 87, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xu, J.; Wang, Z.; Qi, H.; Xu, Q.; Bai, Z.; Zhang, Q.; Bao, K.; Wu, Y.; Zhang, W. 3-(3,4,5 -Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: Microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. [Google Scholar] [CrossRef]
- Wen, Z.; Li, X.; Zuo, D.; Lang, B.; Wu, Y.; Jiang, M.; Ma, H.; Bao, K.; Wu, Y.; Zhang, W. Ultrasound-promoted two-step synthesis of 3-arylselenylindoles and 3-arylthioindoles as novel combretastatin A-4 analogues. Sci. Rep. 2016, 6, 23986–23995. [Google Scholar] [CrossRef]
- Simonetti, S.O.; Larghi, E.L.; Bracca, A.B.J.; Kaufman, T.S. Angular tricyclic benzofurans and related natural products of fungal origin. Isolation, biological activity and synthesis. Nat. Prod. Rep. 2013, 30, 941–969. [Google Scholar] [CrossRef]
- Huang, H.; Qu, Z.; Ji, X.; Deng, G.-J. Three-component bis-heterocycliation for synthesis of 2-aminobenzo [4,5]thieno [3,2-d]thiazoles. Org. Chem. Front. 2019, 6, 1146–1150. [Google Scholar] [CrossRef]
- Radadiya, A.; Shah, A. Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. Eur. J. Med. Chem. 2015, 97, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.H.; Hu, Y.H.; Yang, J.; Liu, T.; Sun, J.; Wang, X.J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- Bhukya, B.; Shukla, A.; Chaturvedi, V.; Trivedi, P.; Kumar, S.; Khan, F.; Negi, A.S.; Srivastava, S.K. Design, synthesis, in vitro and in silico studies of 2, 3-diaryl benzofuran derivatives as antitubercular agents. Bioorg. Chem. 2020, 99, 103784–103793. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef]
- Zeng, S.; Fang, S.; Cai, H.; Wang, D.; Liu, W.; Hu, X.; Sun, P.; Ruan, Z. Selenium-Electrocatalytic Cyclization of 2-Vinylanilides towards Indoles of Peptide Labeling. Chem. Asian J. 2022, e202200762. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pintus, F.; Di Petrillo, A.; Medda, R.; Caria, P.; Matos, M.J.; Vina, D.; Pieroni, E.; Delogu, F.; Era, B.; et al. Novel 2-pheynlbenzofuran derivatives as selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Sci. Rep. 2018, 8, 4424–4436. [Google Scholar] [CrossRef]
- Ma, W.; Kaplaneris, N.; Fang, X.; Gu, L.; Mei, R.; Ackermann, L. Chelation-assisted transition metal-catalysed C–H chalcogenylations. Org. Chem. Front. 2020, 7, 1022–1060. [Google Scholar] [CrossRef]
- He, M.; Gu, L.; Tan, Y.; Wang, Y.; Wang, Y.; Zhang, C.; Ma, W. Palladium-Catalyzed Distal C−H Selenylation of 2-Aryl Acetamides with Diselenides and Selenyl Chlorides. Adv. Synth. Catal. 2020, 362, 5708–5715. [Google Scholar] [CrossRef]
- Bedi, A.; Debnath, S.; Zade, S.S. Diselenolodiselenole: A selenium containing fused heterocycle for conjugated systems. Chem. Commun. 2014, 50, 13454–13456. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Chithiravel, S.; Sharma, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Selenium-Containing Fused Bicyclic Heterocycle Diselenolodiselenole: Field Effect Transistor Study and Structure-Property Relationship. ACS Appl. Mater. Interfaces 2016, 8, 18222–18230. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Zhang, J.R.; Huang, G.B.; Zhou, Y.H.; Chen, Y.Y.; Xu, Y.L. Visible Light-Promoted Selenylation/Cyclization of Enaminones toward the Formation of 3-Selanyl-4H-Chromen-4-Ones. Adv. Synth. Catal. 2021, 363, 1656–1661. [Google Scholar] [CrossRef]
- Godoi, B.; Schumacher, R.F.; Jurinic, C.K.; Belladona, A.L. Diorganyl Dichalcogenides and Copper/Iron Salts: Versatile Cyclization System to Achieve Carbo- and Heterocycles from Alkynes. Synthesis 2021, 53, 2545–2558. [Google Scholar]
- Zeni, G.; Godoi, B.; Jurinic, C.K.; Belladona, A.L.; Schumacher, R.F. Transition Metal-Free Synthesis of Carbo- and Heterocycles via Reaction of Alkynes with Organylchalcogenides. Chem. Rec. 2021, 21, 2880–2895. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Waldo, J.P.; Larock, R.C. Competition Studies in Alkyne Electrophilic Cyclization Reactions. J. Org. Chem. 2009, 74, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Guilarte, V.; Hernando, E.; Sanjuan, A.M. Synthesis of regioselectively functionalized benzo[b]thiophenes by combined ortho-lithiation-halocyclization strategies. J. Org. Chem. 2010, 75, 7443–7446. [Google Scholar] [CrossRef]
- Gay, R.M.; Manarin, F.A.; Schneider, C.C.; Barancelli, D.A.; Costa, M.D.; Zeni, G. FeCl3-Diorganyl Dichalcogenides Promoted Cyclization of 2-Alkynylanisoles to 3-Chalcogen Benzo[b]furans. J. Org. Chem. 2010, 75, 5701–5706. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.S.S.; Iglesias, B.A.; Back, D.F.; Zeni, G. Iron-Promoted Tandem Cyclization of 1,3-Diynyl Chalcogen Derivatives with Diorganyl Dichalcogenides for the Synthesis of Benzo[b]furan-Fused Selenophenes. Adv. Syn. Catal. 2016, 358, 3572–3585. [Google Scholar] [CrossRef]
- Liu, J.; Tian, M.; Li, A.; Ji, L.; Qiu, D.; Zhao, X. Acid-promoted selective synthesis of trifluoromethylselenolated benzofurans with Se-(trifluoromethyl) 4-methylbenzenesulfonoselenoate. Tetrahedron Lett. 2021, 66, 152809–152813. [Google Scholar] [CrossRef]
- Kazmierczak, J.C.; Recchi, A.M.S.; Gritzenco, F.; Balbom, E.B.; Barcellos, T.; Sperança, A.; Godoi, B. Copper-Iodide- and Diorganyl-Diselenide-Promoted Cyclization of 2-Alkynylphenols: Alternative Approach to 3-Organoselanylbenzo[b]furans. Eur. J. Org. Chem. 2017, 2017, 6382–6389. [Google Scholar] [CrossRef]
- An, C.; Li, C.Y.; Huang, X.B.; Gao, W.X.; Zhou, Y.B.; Liu, M.C.; Wu, H.Y. Selenium Radical Mediated Cascade Cyclization: Concise Synthesis of Selenated Benzofurans (Benzothiophenes). Org. Lett. 2019, 21, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Du, H.A.; Zhang, X.G.; Tang, R.Y.; Li, J.H. PdCl2-promoted electrophilic annulation of 2-alkynylphenol derivatives with disulfides or diselenides in the presence of iodine. J. Org. Chem. 2009, 74, 7844–7848. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Cespedes, S.; Ferry, A.; Candish, L.; Glorius, F. Heterogeneously catalyzed direct C-H thiolation of heteroarenes. Angew. Chem. Int. Ed. 2015, 54, 5772–5776. [Google Scholar] [CrossRef]
- Niankai, F.; Ambarneil, S.; Aaron, L.; Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 2017, 357, 575–579. [Google Scholar]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Karkas, M.D. Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev. 2018, 47, 5786–5865. [Google Scholar] [CrossRef]
- Sauermann, N.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Electrocatalytic C–H Activation. ACS Catal. 2018, 8, 7086–7103. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, K.; Zeng, C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Z.; Li, Y.; Kuniyil, R.; Zhang, C.; Ackermann, L.; Ruan, Z. Catalyst-free, direct electrochemical synthesis of annulated medium-sized lactams through C–C bond cleavage. Green Chem. 2020, 22, 1099–1104. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, B.; Levy, L.; Zhang, H.-J.; He, C.; Baran, P.S. Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with a-Heteroatom Phosphorus or Sulfur Alkyl Electrophiles. J. Am. Chem. Soc. 2020, 142, 214–221. [Google Scholar]
- Long, H.; Huang, C.; Zheng, Y.T.; Li, Z.Y.; Jie, L.H.; Song, J.; Zhu, S.; Xu, H.C. Electrochemical C-H phosphorylation of arenes in continuous flow suitable for late-stage functionalization. Nat. Commun. 2021, 12, 6629–6636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fang, Z.; Du, J.; Lin, X.; Liu, C.; He, W.; Yang, Z.; Guo, K. Highly efficient and selective electrochemical synthesis of substituted benzothiophenes and benzofurans in micro-continuous flow. ACS Sustain. Chem. Eng. 2020, 8, 13302–13309. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, J.; Du, J.; Wang, X.; He, W.; Yang, Z.; Liu, C.; Fang, Z.; Guo, K. Oxidant- and Catalyst-Free Synthesis of Sulfonated Benzothiophenes via Electrooxidative Tandem Cyclization. J. Org. Chem. 2021, 86, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, C.; Struwe, J.; Feng, J.; Zhu, G.; Ackermann, L. Electrooxidative dearomatization of biaryls: Synthesis of tri- and difluoromethylated spiro[5.5]trienones. Chem. Sci. 2021, 12, 10092–10096. [Google Scholar] [CrossRef]
- Doerner, C.V.; Scheide, M.R.; Nicoleti, C.R.; Durigon, D.C.; Idiarte, V.D.; Sousa, M.J.A.; Mendes, S.R.; Saba, S.; Neto, J.S.S.; Martins, G.M.; et al. Versatile Electrochemical Synthesis of Selenylbenzo[b]Furan Derivatives Through the Cyclization of 2-Alkynylphenols. Front. Chem. 2022, 10, 880099–880109. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Mo, G.; Zheng, Y.; Zeng, S.; Sun, P.H.; Ruan, Z. Electrochemical Oxidative Phosphorylation of Aldehyde Hydrazones. Org. Lett. 2020, 22, 4016–4020. [Google Scholar] [CrossRef]
- Cheng, X.; Hasimujiang, B.; Xu, Z.; Cai, H.; Chen, G.; Mo, G.; Ruan, Z. Direct Electrochemical Selenylation/Cyclization of Alkenes: Access to Functionalized Benzheterocycles. J. Org. Chem. 2021, 86, 16045–16058. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Mo, G.; Jiang, W.; Zheng, C.; Feng, P.; Ruan, Z. Direct Electrochemical Synthesis of Sulfur-Containing Triazolium Inner Salts. Chin. J. Chem. 2021, 39, 942–946. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, X.; Hasimujiang, B.; Xu, Z.; Li, F.; Ruan, Z. Electrochemical regioselective C–H selenylation of 2H-indazole derivatives. Org. Biomol. Chem. 2021, 20, 117–121. [Google Scholar] [CrossRef]
- Ruan, Z.; Huang, Z.; Xu, Z.; Zeng, S.; Feng, P.; Sun, P.-H. Late-stage azolation of benzylic C–H bonds enabled by electrooxidation. Sci. China Chem. 2021, 64, 800–807. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Y.F.; Huang, X.B.; Gao, W.X.; Zhou, Y.B.; Liu, M.C.; Wu, H.Y. Three-Component Reactions of Alkynone o-Methyloximes, Element Selenium, and Boronic Acids Leading to 4-Organoselenylisoxazoles. ACS Omega. 2020, 5, 23358–23363. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Alhumade, H.; Yi, H.; Cai, H.; Lei, A. Electrochemical Radical Selenylation of Alkenes and Arenes via Se–Se Bond Activation. Org. Lett. 2021, 23, 7724–7729. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Richard, C. Larock, Synthesis of 2,3-Disubstituted Benzo[b]furans by the Palladium-Catalyzed Coupling of o-Iodoanisoles and Terminal Alkynes, Followed by Electrophilic Cyclization. J. Org. Chem. 2005, 70, 10292–10296. [Google Scholar] [CrossRef] [PubMed]
- Xavier, D.F.; Carpe, M.; Sandagorda, A.; Martarelo, E.; Neto, S.; Sebastião, J.; Schumacher, R.F.; Silva, M.S. Synthesis of 3-selanylbenzo[b]furans promoted by SelectFluor. RSC Adv. 2020, 10, 13975–13983. [Google Scholar] [CrossRef]
- Perin, G.; Soares, L.K.; Hellwig, P.S.; Silva, M.S.; Neto, J.S.S.; Roehrs, J.A.; Barcellos, T.; Lenardão, E.J. Synthesis of 2,3-bis-organochalcogenyl-benzo[b]chalcogenophenes promoted by Oxone®. New J. Chem. 2019, 43, 6323–6331. [Google Scholar] [CrossRef]
- Murata, Y.; Otake, N.; Sano, M.; Matsumura, M.; Yasuike, S. Copper-catalyzed C−H Selenation of 2-Substituted Benzo[b]furans with Diaryl Diselenides: Synthesis of 2-Substituted 3-Selanylbenzo[b]furan Derivatives. Asian J. Org. Chem. 2021, 10, 2975–2981. [Google Scholar] [CrossRef]








 | ||
|---|---|---|
| Entry | Deviation from Standard Conditions | Yield (%) b |
| 1 | none | 96 |
| 2 | MeOH as solvent (6 h) | 76 |
| 3 | MeCN as solvent (6 h) | 92 |
| 4 | n-Bu4NClO4 instead of n-Bu4NPF6 | 85 |
| 5 | n-Bu4NBF4 instead of n-Bu4NPF6 | 85 |
| 6 | LiClO4 instead of n-Bu4NPF6 | 75 |
| 7 | 5 mA instead of 10 mA (6 h) | 87 |
| 8 | C(+)-C(-) | 85 |
| 9 | Pt plate as anode | 93 |
| 10 | 2a (0.18 mmol) instead of 2a (0.36 mmol) | 81 |
| 11 | no electrolyte | 0 |
| 12 | no electric current | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasimujiang, B.; Lin, S.; Zheng, C.; Zeng, Y.; Ruan, Z. Direct Electrooxidative Selenylation/Cyclization of Alkynes: Access to Functionalized Benzo[b]furans. Molecules 2022, 27, 6314. https://doi.org/10.3390/molecules27196314
Hasimujiang B, Lin S, Zheng C, Zeng Y, Ruan Z. Direct Electrooxidative Selenylation/Cyclization of Alkynes: Access to Functionalized Benzo[b]furans. Molecules. 2022; 27(19):6314. https://doi.org/10.3390/molecules27196314
Chicago/Turabian StyleHasimujiang, Balati, Shengsheng Lin, Chengwei Zheng, Yong Zeng, and Zhixiong Ruan. 2022. "Direct Electrooxidative Selenylation/Cyclization of Alkynes: Access to Functionalized Benzo[b]furans" Molecules 27, no. 19: 6314. https://doi.org/10.3390/molecules27196314
APA StyleHasimujiang, B., Lin, S., Zheng, C., Zeng, Y., & Ruan, Z. (2022). Direct Electrooxidative Selenylation/Cyclization of Alkynes: Access to Functionalized Benzo[b]furans. Molecules, 27(19), 6314. https://doi.org/10.3390/molecules27196314