The Effects of Polyphenol, Tannic Acid, or Tannic Acid in Combination with Pamidronate on Human Osteoblast Cell Line Metabolism
Abstract
:1. Introduction
2. Results
2.1. EC50 of TA and Combination of TA with PAM on hFOB 1.19 Cells
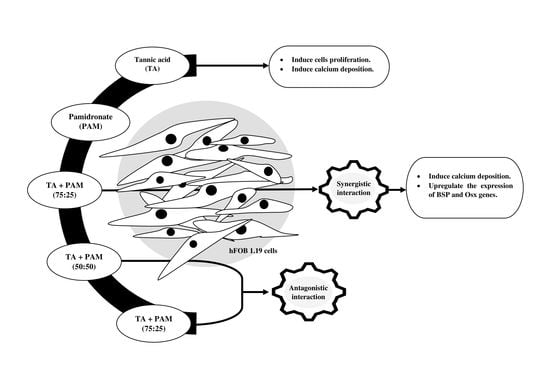
2.2. Synergistic Activity of TA and PAM
2.3. Proliferative Activity of hFOB 1.19 Cells
2.4. Formation of Mineralized Calcium and Phosphate Deposits
2.5. Expression of BSP and Osx Genes
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.1.1. Cell Revival and Subculture
4.1.2. Preparation for Cell Treatment
4.2. MTT Assay
4.3. Analysis of Synergistic Activity
4.4. Trypan Blue Exclusion Assay
4.5. Histochemical Assays for Mineralized Calcium and Phosphate Deposits
4.5.1. Alizarin Red S Staining
4.5.2. Von Kossa Staining
4.6. Gene Expression by Polymerase Chain Reaction (PCR)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care 2017, 26, e12740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-osteoclast communication and bone homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, G.K.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Role of glutamic acid-rich sequences in the nucleation of hydroxyapatite by bone sialoprotein. Biochem. J. 1994, 302, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Tang, W.; Li, Y.; Yang, F.; Dowd, D.R.; MacDonald, P.N. Osteoblast-specific transcription factor osterix increases vitamin d receptor gene expression in osteoblasts. PLoS ONE 2011, 6, e26504. [Google Scholar] [CrossRef]
- Chang, C.Y.; Rosenthal, D.I.; Mitchell, D.M.; Handa, A.; Kattapuram, S.V.; Huang, A.J. Imaging findings of metabolic bone disease. RadioGraphics 2016, 36, 1871–1887. [Google Scholar] [CrossRef]
- Daroszewska, A. Prevention and treatment of osteoporosis in women: An update. Obstet. Gynaecol. Reprod. Med. 2015, 25, 181–187. [Google Scholar] [CrossRef]
- Koch, F.P.; Yekta, S.S.; Merkel, C.; Ziebart, T.; Smeets, R. The impact of bisphosphonates on the osteoblast proliferation and Collagen gene expression in vitro. Head Face Med. 2010, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Pazianas, M.; Abrahamsen, B.; Ferrari, S.; Russell, R.G. Eliminating the need for fasting with oral administration of bisphosphonates. Ther. Clin. Risk Manag. 2013, 9, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Kim-Sooi, L.; Lean-Keng, S. Herbal medicines: Malaysian women’s knowledge and practice. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Hubert, P.; Lee, S.; Lee, S.K.; Chun, O. Dietary polyphenols, berries, and age-related bone loss: A review based on human, animal, and cell studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Offord, E. Naturally plant-derived compounds: Role in bone anabolism. Curr. Mol. Pharmacol. 2012, 5, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
- Ko, C.H.; Siu, W.S.; Wong, H.L.; Gao, S.; Shum, W.T.; Lau, C.P.; Cheng, S.W.; Tam, J.C.; Hung, L.K.; Fung, K.P.; et al. In vivo study on the pharmacological interactions between a chinese herbal formula ELP and antiresorptive drugs to counteract osteoporosis. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Nidhi, S. Concept of drug interaction. Int. Res. J. Pharm. 2012, 3, 120–122. [Google Scholar]
- Abdullah, A.R.; Hapidin, H.; Abdullah, H. The role of semipurified fractions isolated from Quercus infectoria on bone metabolism by using hFOB 1.19 human fetal osteoblast cell model. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudhah, A.; Hermizi, H.; Hasmah, A. Combination treatment of bisphosphonate (pamidronate) and Quercus infectoria semi-purified fraction promotes proliferation and differentiation of osteoblast cell via expression of Osterix and Runx2 marker. Asian Pac. J. Trop. Biomed. 2018, 8, 261–267. [Google Scholar]
- Hapidin, H.; Romli, N.A.A.; Abdullah, H. Proliferation study and microscopy evaluation on the effects of tannic acid in human fetal osteoblast cell line (hFOB 1.19). Microsc. Res. Tech. 2019, 82, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Leiper, K.A.; Miedl, M. Colloidal stability of beer. In Handbook of Alcoholic Beverages Series, Beer: A Quality Perspective, 1st ed.; Charles, B., Inge, R., Graham, S., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 111–161. [Google Scholar]
- Coşan, T.D.; Saydam, F.; Özbayer, C.; Doğaner, F.; Soyocak, A.; Güneş, H.V.; Değirmenci, İ.; Kurt, H.; Üstüner, M.C.; Bal, C. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology 2015, 67, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.L.; von-Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and dietary phytochemicals in bone protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef]
- García-Martínez, O.; De-Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic compounds in extra virgin olive oil stimulate human osteoblastic cell proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Tomczyk, A.; Muszyński, S. The effect of tannic acid on the bone tissue of adult male Wistar rats exposed to cadmium and lead. Exp. Toxicol. Pathol. 2017, 69, 131–141. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Herrera-Briones, F.J.; Bassam, T.; Vallecillo-Capilla, M.F.; Reyes-Botella, C. Factors affecting dental implant stability measured using the ostell mentor device. Implant Dent. 2015, 24, 565–577. [Google Scholar] [CrossRef]
- Cokol, M.; Chua, H.N.; Tasan, M.; Mutlu, B.; Weinstein, Z.B.; Suzuki, Y.; Nergiz, M.E.; Costanzo, M.; Baryshnikova, A.; Giaever, G.; et al. Systematic exploration of synergistic drug pairs. Mol. Syst. Biol. 2011, 7, 544. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid. Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [Green Version]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, S23–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonjour, J.P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 2011, 30, 438S–448S. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Brown, S.E.; Krebsbach, P.H. Human embryonic stem cells undergo osteogenic differentiation in human bone marrow stromal cell microenvironments. J. Stem Cells 2007, 2, 139–147. [Google Scholar] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, C.; Yamazaki, D.; Sato, M.; Takeshima, H.; Memtily, N.; Hatano, Y.; Tsukuba, T.; Sakai, E. Calcium phosphate mineralization in bone tissues directly observed in aqueous liquid by atmospheric SEM (ASEM) without staining: Microfluidics crystallization chamber and immuno-EM. Sci. Rep. 2019, 9, 7352. [Google Scholar] [CrossRef]
- Tian, X.; Yuan, X.; Feng, D.; Wu, M.; Yuan, Y.; Ma, C.; Xie, D.; Guo, J.; Liu, C.; Lu, Z. In vivo study of polyurethane and tannin-modified hydroxyapatite composites for calvarial regeneration. J. Tissue Eng. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Bleve, G.; Rizzotti, L.; Dellaglio, F.; Torriani, S. Development of Reverse Transcription (RT)-PCR and Real-Time RT-PCR assays for rapid detection and quantification of viable yeasts and molds contaminating yogurts and pasteurized food products. Appl. Environ. Microbiol. 2003, 69, 4116–4122. [Google Scholar] [CrossRef] [Green Version]
- Rebouças, E.D.L.; Costa, J.J.D.N.; Passos, M.J.; Passos, J.R.D.S.; Hurk, R.V.D.; Silva, J.R.V. Real time PCR and importance of housekeepings genes for normalization and quantification of mRNA expression in different tissues. Braz. Arch. Biol. Technol. 2013, 56, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Trzeciakiewicz, A.; Habauzit, V.; Horcajada, M.N. When nutrition interacts with osteoblast function: Molecular mechanisms of polyphenols. Nutr. Res. Rev. 2009, 22, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Jahanian, E.; Karimifar, M.; Rafieian-Kopaei, M. Antioxidants as a novel way to alleviate the adverse effects of oxidative stress in osteoporosis. J. Parathyr. Dis. 2016, 4, 60–65. [Google Scholar]
- Yang, Y.; Huang, Y.; Zhang, L.; Zhang, C. Transcriptional regulation of bone sialoprotein gene expression by Osx. Biochem. Biophys. Res. Commun. 2016, 476, 574–579. [Google Scholar] [CrossRef]
- Wang, S.; Sasaki, Y.; Zhou, L.; Matsumura, H.; Araki, S.; Mezawa, M.; Takai, H.; Chen, Z.; Ogata, Y. Transcriptional regulation of bone sialoprotein gene by interleukin-11. Gene 2011, 476, 46–55. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Feng, J.Q.; Dusevich, V.M.; Sinha, K.; Zhang, H.; Darnay, B.G.; de-Crombrugghe, B. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc. Natl. Acad. Sci. USA 2010, 107, 12919–12924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Shi, K.; Wang, L.; Lu, J.; Li, H.; Pan, S.; Ma, C. Characterization of Osterix protein stability and physiological role in osteoblast differentiation. PLoS ONE 2013, 8, e56451. [Google Scholar] [CrossRef]
- Wang, C.; Liao, H.; Cao, Z. Role of osterix and microRNAs in bone formation and tooth development. Med. Sci. Monit. 2016, 22, 2934–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar] [PubMed]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent advances of osterix transcription factor in osteoblast differentiation and bone formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef] [PubMed]
- Ponader, S.; Brandt, H.; Vairaktaris, E.; von-Wilmowsky, C.; Nkenke, E.; Schlegel, K.A.; Neukam, F.W.; Holst, S.; Müller, F.; Greil, P. In vitro response of hFOB cells to pamidronate modified sodium silicate coated cellulose scaffolds. Colloids Surf. B Biointerfaces 2008, 64, 275–283. [Google Scholar] [CrossRef]
- Hassan, M.A.; Azemin, W.A.; Dharmaraj, S.; Mohd, K.S. Cytotoxic effect of hepcidin (TH1-5) on human breast cancer cell line (MCF7). J. Teknol. 2015, 77, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Putnam, S.E.; Scutt, A.M.; Bicknell, K.; Priestley, C.M.; Williamson, E.M. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phyther. Res. 2007, 21, 99–112. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Grady-Gunn, W.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Brauer, A.; Pohlemann, T.; Metzger, W. Osteogenic differentiation of immature osteoblasts: Interplay of cell culture media and supplements. Biotech. Histochem. 2016, 91, 161–169. [Google Scholar] [CrossRef]






| Treatment | TA | PAM | 50:50 (TA:PAM) | 25:75 (TA:PAM) | 75:25 (TA:PAM) |
|---|---|---|---|---|---|
| EC50 (µg/mL) | 0.56 | 15.27 | 2.25 | 3.80 | 0.48 |
| Log10 EC50 | −0.25 | 1.18 | 0.35 | 0.58 | −0.32 |
| Combination Ratio (TA:PAM) | Combination Index (CI) | Indication |
|---|---|---|
| 50:50 | 2.0826 | Antagonism |
| 25:75 | 1.8831 | Antagonism |
| 75:25 | 0.6372 | Synergism |
| Gene | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| Bone sialoprotein (BSP) | AATGAAAACGAAGAAAGCGAAG | ATCATAGCCATCGTAGCCTTGT |
| Osterix (Osx) | TGCGAAGCCTTGCCATACA | TCCTCCTGCGACTGCCCTAA |
| β-actin | GGCATCGTGATGGACTCCG | GCTGGAAGGTGGACAGCGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapidin, H.; Hashim, N.M.; Kasiram, M.Z.; Abdullah, H. The Effects of Polyphenol, Tannic Acid, or Tannic Acid in Combination with Pamidronate on Human Osteoblast Cell Line Metabolism. Molecules 2022, 27, 451. https://doi.org/10.3390/molecules27020451
Hapidin H, Hashim NM, Kasiram MZ, Abdullah H. The Effects of Polyphenol, Tannic Acid, or Tannic Acid in Combination with Pamidronate on Human Osteoblast Cell Line Metabolism. Molecules. 2022; 27(2):451. https://doi.org/10.3390/molecules27020451
Chicago/Turabian StyleHapidin, Hermizi, Nor Munira Hashim, Mohamad Zahid Kasiram, and Hasmah Abdullah. 2022. "The Effects of Polyphenol, Tannic Acid, or Tannic Acid in Combination with Pamidronate on Human Osteoblast Cell Line Metabolism" Molecules 27, no. 2: 451. https://doi.org/10.3390/molecules27020451
APA StyleHapidin, H., Hashim, N. M., Kasiram, M. Z., & Abdullah, H. (2022). The Effects of Polyphenol, Tannic Acid, or Tannic Acid in Combination with Pamidronate on Human Osteoblast Cell Line Metabolism. Molecules, 27(2), 451. https://doi.org/10.3390/molecules27020451