Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities
Abstract
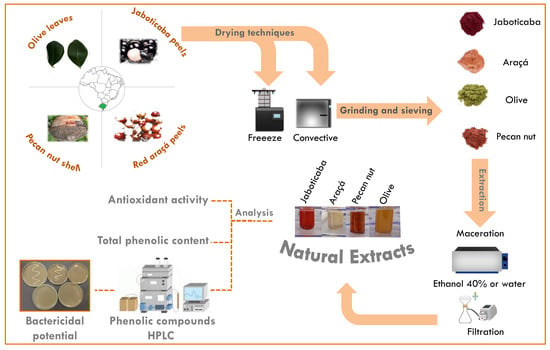
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity
2.2. Total Phenolic Compounds
2.3. Phenolic Compounds Analysis by High-Performance Liquid Chromatography—HPLC
2.4. Microbiological Analysis
2.5. Main Findings and Future Research Directions
- (i)
- The extracts obtained from agro-industrial residues in the Rio Grande do Sul, Brazil, presented competitive results compared to the literature. This trend is extremely relevant since the Rio Grande do Sul is historically characterized by high harvesting activity and other processes involved in the food industry, which generates a huge volume of waste [11].
- (ii)
- The freeze-drying process has an advantage over the conservation of bioactive compounds from plant samples. However, it is more expensive and time-consuming [27]. Therefore, the superior results using the convective-drying obtained for olive leaf extract and pecan nut shell extract present a better and more economical alternative for producing extracts from these biomasses.
- (iii)
- Despite the promising results regarding the variables extractor solvent (water or ethanol 40%) and drying method (convective-drying and freeze-drying), other variables can be evaluated in future works, such as type of extraction, temperature, and pH.
- (iv)
- Although the pecan nut shell extract showed higher values of TP than the other extracts studied in this work, all extracts have relevant results that allow their application in several areas, such as pharmaceuticals, cosmetics, food packaging, and medicine, among others.
- (v)
- The results obtained in this work, using water or ethanol (40%) as extracting solvent, have great potential for industrial use since there are few restrictions since they are green solvents.
- (vi)
- Byproducts can be used to complement or create new products with health and technological benefits through application in the food, pharmaceutical, and cosmetic industries. For example, processing jaboticaba peel, olives leaves, araçá peel, and pecan nut shells takes advantage of these raw materials to develop innovative and healthier products. In addition, using byproducts can promote more efficient use of natural resources. However, specific in vitro, in vivo, and clinical trials must be performed to confirm the benefits to human health or attest to these biomasses as functional or nutraceuticals.
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation and Extraction Procedure
3.3. Extracts Characterization
3.3.1. Antioxidant Activity
3.3.2. Determination of Total Phenolic Compounds
3.3.3. Phenolic Compounds Analysis by High-Performance Liquid Chromatography—HPLC
3.3.4. Microbiological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Moalla, S.; Ammar, I.; Fauconnier, M.-L.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Development and Characterization of Chitosan Films Carrying Artemisia Campestris Antioxidants for Potential Use as Active Food Packaging Materials. Int. J. Biol. Macromol. 2021, 183, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Araújo, R.; Martínez-Vázquez, D.G.; Charles-Rodríguez, A.V.; Rangel-Ortega, S.; Robledo-Olivo, A. Bioactive Compounds from Agricultural Residues, Their Obtaining Techniques, and the Antimicrobial Effect as Postharvest Additives. Int. J. Food Sci. 2021, 2021, 9936722. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of Bioactive Compounds: A Review. Nat. Products Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Tsiaka, T.; Sinanoglou, V.J.; Zoumpoulakis, P. Extracting Bioactive Compounds from Natural Sources Using Green High-Energy Approaches: Trends and Opportunities in Lab- and Large-Scale Applications. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 307–365. [Google Scholar]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 2663. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial Mutations and Mitoepigenetics: Focus on Regulation of Oxidative Stress-Induced Responses in Breast Cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Valério Filho, A.; Tholozan, L.V.; da Silva, E.O.; Meili, L.; de Almeida, A.R.F.; da Rosa, G.S. Perspectives of the Reuse of Agricultural Wastes from the Rio Grande Do Sul, Brazil, as New Adsorbent Materials. In Biomass-Derived Materials for Environmental Applications; Anastopoulos, I., Lima, E.C., Meili, L., Giannakoudakis, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–266. ISBN 9780323919142. [Google Scholar]
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schünemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A Fragile Biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Olive Tree (Olea europaea L.) Leaf as a Waste by-Product of Table Olive and Olive Oil Industry: A Review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G.; Gerasopoulos, D. Valorization of Olive Leaves: Spray Drying of Olive Leaf Extract. Waste Biomass Valorization 2018, 9, 619–633. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of Bioactive Compounds in Commercial Olive Leaf Extracts, and Olive Leaves and Their Infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef] [Green Version]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Benali, T.; Rharrabti, Y. Antibacterial and Antioxidant Potentials of Phenolic Extracts from Olive Mill Wastewater and Their Use to Enhance the Stability of Olive Oil. Riv. Ital. Delle Sostanze Grasse 2020, 97, 31–42. [Google Scholar]
- Inada, K.O.P.; Silva, T.B.R.; Lobo, L.A.; Domingues, R.M.C.P.; Perrone, D.; Monteiro, M. Bioaccessibility of Phenolic Compounds of Jaboticaba (Plinia jaboticaba) Peel and Seed after Simulated Gastrointestinal Digestion and Gut Microbiota Fermentation. J. Funct. Foods 2020, 67, 103851. [Google Scholar] [CrossRef]
- Dos Santos Pereira, E.; Vinholes, J.R.; Camargo, T.M.; Nora, F.R.; Crizel, R.L.; Chaves, F.; Nora, L.; Vizzotto, M. Characterization of Araçá Fruits (Psidium cattleianum Sabine): Phenolic Composition, Antioxidant Activity and Inhibition of α-Amylase and α-Glucosidase. Food Biosci. 2020, 37, 100665. [Google Scholar] [CrossRef]
- Gomes, A.C.A.; da Costa Lima, M.; de Oliveira, K.Á.R.; dos Santos Lima, M.; Magnani, M.; Câmara, M.P.S.; de Souza, E.L. Coatings with Chitosan and Phenolic-Rich Extract from Acerola (Malpighia emarginata D.C.) or Jabuticaba (Plinia jaboticaba (Vell.) Berg) Processing by-Product to Control Rot Caused by Lasiodiplodia Spp. in Papaya (Carica papaya L.) Fruit. Int. J. Food Microbiol. 2020, 331, 108694. [Google Scholar] [CrossRef]
- Kureck, I.; de Brito Policarpi, P.; Toaldo, I.M.; de Oliveira Brisola Maciel, M.V.; Bordignon-Luiz, M.T.; Barreto, P.L.M.; Block, J.M. Chemical Characterization and Release of Polyphenols from Pecan Nut Shell [Carya illinoinensis (Wangenh) C. Koch] in Zein Microparticles for Bioactive Applications. Plant Foods Hum. Nutr. 2018, 73, 137–145. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic Compounds and Antioxidant Activity of Kernels and Shells of Mexican Pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef]
- Pinheiro do Prado, A.C.; Manion, B.A.; Seetharaman, K.; Deschamps, F.C.; Barrera Arellano, D.; Block, J.M. Relationship between Antioxidant Properties and Chemical Composition of the Oil and the Shell of Pecan Nuts [Caryaillinoinensis (Wangenh) C. Koch]. Ind. Crops Prod. 2013, 45, 64–73. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Urrea-López, R.; de la Rosa, L.A. Bioactive Components and Health Effects of Pecan Nuts and Their Byproducts: A Review. J. Food Bioact. 2018, 1, 56–92. [Google Scholar] [CrossRef]
- Fleck, N.; Sant Anna, V.; Oliveira, W.d.C.; Brandelli, A.; Fonseca Veras, F. Jaboticaba Peel Extract as an Antimicrobial Agent: Screening and Stability Analysis. Br. Food J. 2021, 124, 2793–2804. [Google Scholar] [CrossRef]
- Bezerra, F.W.F.; de Oliveira, M.S.; Bezerra, P.N.; Cunha, V.M.B.; Silva, M.P.; da Costa, W.A.; Pinto, R.H.H.; Cordeiro, R.M.; da Cruz, J.N.; Chaves Neto, A.M.J.; et al. Extraction of Bioactive Compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Asiri, I.A.M., Isloor, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–167. [Google Scholar]
- Avila, L.B.; Fontes, M.R.V.; da Rosa Zavareze, E.; Moraes, C.C.; Morais, M.M.; da Rosa, G.S. Recovery of Bioactive Compounds from Jaboticaba Peels and Application into Zein Ultrafine Fibers Produced by Electrospinning. Polymers 2020, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Ah-Hen, K.; Vega-Gálvez, A.; Honores, C.; Moraga, N.O. Stevia Rebaudiana Leaves: Effect of Drying Process Temperature on Bioactive Components, Antioxidant Capacity and Natural Sweeteners. Plant Foods Hum. Nutr. 2016, 71, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of Drying Methods on the Retention of Bioactive Compounds in African Eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Effects of Different Drying Methods on the Bioactive Compounds and Antioxidant Properties of Edible Centaurea (Centaurea cyanus) Petals. Braz. J. Food Technol. 2018, 21, 327–332. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927. [Google Scholar] [CrossRef]
- Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of Microwave, Ultrasonic and Conventional Techniques for Extraction of Bioactive Compounds from Olive Leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Hassan, N.D.; Mamat, S.N.H.; Nawi, N.M.; Rashid, W.A.; Tan, N.A. Extraction Technologies and Solvents of Phytocompounds from Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds from Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 523–560. [Google Scholar]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of Bioactive Compounds from Mango (Mangifera indica L. Var. Carabao) Seed Kernel with Ethanol–Water Binary Solvent Systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of Age and Extraction Solvent on Phytochemical Content and Antioxidant Activity of Fresh Moringa oleifera L. Leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Meira, N.A.N.; Pereira, N.P.; Maciel, L.F.; Menezes-Filho, J.A.; Oliveira, S.S.P. Development and Stability Testing of Emulsions with Myrciaria cauliflora (Jaboticaba) Peel Extracts for Cosmetic Application. J. Cosmetol. 2018, 2, 000106. [Google Scholar]
- Pitz, H.D.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell. Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef] [Green Version]
- Salvador, A.A.; Block, J.M.; Ferreira, S.R.S. Supercritical Fluid Extraction of Byproduct from Pecan [Caryaillinoinensis (Wangenh) K. Koch] Oil Industry. In Proceedings of the III Iberoamerican Conference on Supercritical Fluids, Cartagena de Indias, Colombia, 1–5 April 2013; pp. 1–7. [Google Scholar]
- Meregalli, M.M.; Puton, B.M.S.; Camera, F.D.; Amaral, A.U.; Zeni, J.; Cansian, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and Ultrasound-Assisted Methods for Extraction of Bioactive Compounds from Red Araçá Peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze Dried Extract from Olive Leaves: Valorisation, Extraction Kinetics and Extract Characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Denardin, C.C.; Hirsch, G.E.; da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.F.; Guma, F.T.C.R.; Emanuelli, T. Antioxidant Capacity and Bioactive Compounds of Four Brazilian Native Fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. Antioxidant Activities of Bambara Groundnuts as Assessed by FRAP and DPPH Assays. Am. J. Food Nutr. 2015, 3, 7–11. [Google Scholar] [CrossRef]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga-España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A Novel Biodegradable Film Based on κ-Carrageenan Activated with Olive Leaves Extract. Food Sci. Nutr. 2020, 8, 3147–3156. [Google Scholar] [CrossRef]
- Lenquiste, S.A.; da Silva Marineli, R.; Moraes, É.A.; Dionísio, A.P.; de Brito, E.S.; Maróstica, M.R. Jaboticaba Peel and Jaboticaba Peel Aqueous Extract Shows in Vitro and in Vivo Antioxidant Properties in Obesity Model. Food Res. Int. 2015, 77, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Salvador, A.A.; Podestá, R.; Block, J.M.; Ferreira, S.R.S. Increasing the Value of Pecan Nut [Carya illinoinensis (Wangenh) C. Koch] Cake by Means of Oil Extraction and Antioxidant Activity Evaluation. J. Supercrit. Fluids 2016, 116, 215–222. [Google Scholar] [CrossRef]
- Bittencourt, G.M.; Firmiano, D.M.; Fachini, R.P.; Lacaz-Ruiz, R.; Fernandes, A.M.; Oliveira, A.L. Application of Green Technology for the Acquisition of Extracts of Araçá (Psidium grandifolium Mart. Ex DC.) Using Supercritical CO2 and Pressurized Ethanol: Characterization and Analysis of Activity. J. Food Sci. 2019, 84, 1297–1307. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic Compounds, Antioxidant, and Antibacterial Properties of Pomace Extracts from Four Virginia-Grown Grape Varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Baseggio, A.M.; Angolini, C.F.F.; Pastore, G.M.; Cazarin, C.B.B.; Marostica-Junior, M.R. Influence of Different Types of Acids and PH in the Recovery of Bioactive Compounds in Jabuticaba Peel (Plinia cauliflora). Food Res. Int. 2019, 124, 16–26. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the Solvents on the Extraction of Major Phenolic Compounds (Punicalagin, Ellagic Acid and Gallic Acid) and Their Antioxidant Activities in Pomegranate Aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef] [Green Version]
- Andrade Neves, N.; César Stringheta, P.; Ferreira da Silva, I.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification and Quantification of Phenolic Composition from Different Species of Jabuticaba (Plinia spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021, 355, 129605. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Nunes, S.; Martínez-Blázquez, J.A.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Effect of High Hydrostatic Pressure and Drying Methods on Phenolic Compounds Profile of Jabuticaba (Myrciaria jaboticaba) Peel and Seed. Food Chem. 2020, 309, 125794. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Anthocyanin-Rich Extract of Jabuticaba Epicarp as a Natural Colorant: Optimization of Heat- and Ultrasound-Assisted Extractions and Application in a Bakery Product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef]
- Moróstica-Junior, M.R.; Alves Cagnon Quitete, V.H.; De Almeida Lamas, C.; Alves Lenquiste, S.; Reyes Reyes, F.G.; Aparecida De Campos Braga, P.; Mara Baseggio, A. Composição Compreendendo Extrato da Casca de Jabuticaba, e Usos do Mesma. WO/2018/165726, 30 October 2018. [Google Scholar]
- Biegelmeyer, R.; Andrade, J.M.M.; Aboy, A.L.; Apel, M.A.; Dresch, R.R.; Marin, R.; do Carmo Bassols Raseira, M.; Henriques, A.T. Comparative Analysis of the Chemical Composition and Antioxidant Activity of Red (Psidium cattleianum) and Yellow (Psidium cattleianum Var. Lucidum) Strawberry Guava Fruit. J. Food Sci. 2011, 76, C991–C996. [Google Scholar] [CrossRef]
- Vieira, L.M.; Marinho, L.M.G.; Rocha, J.d.C.G.; Barros, F.A.R.; Stringheta, P.C. Chromatic Analysis for Predicting Anthocyanin Content in Fruits and Vegetables. Food Sci. Technol. 2019, 39, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health Benefits of Cyanidin-3-Glucoside as a Potent Modulator of Nrf2-Mediated Oxidative Stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-QTOF-MS as a Powerful Analytical Tool for Characterising Phenolic Compounds in Olive-Leaf Extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of Phenolic Compounds and Mannitol in Olive Leaves Extracts from Six Spanish Cultivars: Extraction with the Soxhlet Method and Pressurized Liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, A.C.P.; da Silva, H.S.; da Silveira, S.M.; Barreto, P.L.M.; Vieira, C.R.W.; Maraschin, M.; Ferreira, S.R.S.; Block, J.M. Effect of the Extraction Process on the Phenolic Compounds Profile and the Antioxidant and Antimicrobial Activity of Extracts of Pecan Nut [Carya illinoinensis (Wangenh) C. Koch] Shell. Ind. Crops Prod. 2014, 52, 552–561. [Google Scholar] [CrossRef]
- Hilbig, J.; de Britto Policarpi, P.; de Souza Grinevicius, V.M.A.; Mota, N.S.R.S.; Toaldo, I.M.; Luiz, M.T.B.; Pedrosa, R.C.; Block, J.M. Aqueous Extract from Pecan Nut [Carya illinoinensis (Wangenh) C. Koch] Shell Show Activity against Breast Cancer Cell Line MCF-7 and Ehrlich Ascites Tumor in Balb-C Mice. J. Ethnopharmacol. 2018, 211, 256–266. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Darvishzadeh, P.; Orsat, V. Microwave-Assisted Extraction of Antioxidant Compounds from Russian Olive Leaves and Flowers: Optimization, HPLC Characterization and Comparison with Other Methods. J. Appl. Res. Med. Aromat. Plants 2022, 27, 100368. [Google Scholar] [CrossRef]
- Gould, D. Effective Strategies for Prevention and Control of Gram-Negative Infections. Nurs. Stand. 2009, 23, 42–46. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an Alternative to Antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial Properties of Phenolic Compounds from Berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Costa, M.R.; Pereira, M.F.; Pereira, J.O.; Soares, J.C.; Pintado, M.M. Aqueous Extracts of Vaccinium corymbosum as Inhibitors of Staphylococcus aureus. Food Control 2015, 51, 314–320. [Google Scholar] [CrossRef]
- Quave, C.L.; Lyles, J.T.; Kavanaugh, J.S.; Nelson, K.; Parlet, C.P.; Crosby, H.A.; Heilmann, K.P.; Horswill, A.R. Castanea Sativa (European chestnut) Leaf Extracts Rich in Ursene and Oleanene Derivatives Block Staphylococcus Aureus Virulence and Pathogenesis without Detectable Resistance. PLoS ONE 2015, 10, e0136486. [Google Scholar] [CrossRef] [Green Version]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The Antibacterial Activity of Coriolus versicolor Methanol Extract and Its Effect on Ultrastructural Changes of Staphylococcus aureus and Salmonella enteritidis. Front. Microbiol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Francine, U.; Jeannette, U.; Jean Pierre, R. Assessment of Antibacterial Activity of Neem Plant (Azadirachta indica) on Staphylococcus aureus and Escherichia coli. J. Med. Plants Stud. JMPS 2015, 85, 85–91. [Google Scholar]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The Antibacterial Activity of Extracts of Nine Plant Species with Good Activity against Escherichia coli against Five Other Bacteria and Cytotoxicity of Extracts. BMC Complement. Altern. Med. 2017, 17, 133. [Google Scholar] [CrossRef] [Green Version]
- Flores-Estrada, R.A.; Gámez-Meza, N.; Medina-Juárez, L.A.; Castillón-Campaña, L.G.; Molina-Domínguez, C.C.; Rascón-Valenzuela, L.A.; García-Galaz, A. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of Wastes from Pecan Nut [Carya illinoinensis (Wagenh) K. Koch]. Waste Biomass Valorization 2020, 11, 3419–3432. [Google Scholar] [CrossRef]
- De Lima, A.S.; Maia, D.V.; Haubert, L.; Oliveira, T.L.; Fiorentini, Â.M.; Rombaldi, C.V.; da Silva, W.P. Action Mechanism of Araçá (Psidium cattleianum Sabine) Hydroalcoholic Extract against Staphylococcus aureus. LWT 2020, 119, 108884. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the In-Vitro Antimicrobial Activity of Oleuropein and Hydroxytyrosol. J. Pharm. Pharmacol. 2010, 51, 971–974. [Google Scholar] [CrossRef]
- Guillermo Avila, J.; de Liverant, J.G.; Martínez, A.; Martínez, G.; Muñoz, J.L.; Arciniegas, A.; Romo de Vivar, A. Mode of Action of Buddleja cordata Verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic Interactions between Phenolic Compounds Identified in Grape Pomace Extract with Antibiotics of Different Classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Environ. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.C.; de Souza, P.K.; Silva, B.D.Z.; Martiny, T.R.; Moraes, C.C.; Morais, M.M.; Raghavan, V.; da Rosa, G.S. Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging. Molecules 2020, 25, 5563. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- NCCLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 6th ed.; NCCLS: Berwyn, IL, USA, 2003; Volume 23, ISBN 1562384864. [Google Scholar]

| Extract | FW | FE | CW | CE |
|---|---|---|---|---|
| Jaboticaba peel | 93.21 ± 0.07 a | 92.01 ± 0.73 a | 57.77 ± 0.56 b | 91.28 ± 0.29 a |
| Olive leaf | 95.23 ± 1.81 a | 93.05 ± 0.42 a | 91.89 ± 0.02 a | 94.85 ± 0.02 a |
| Araçá peel | 59.85 ± 1.49 c | 82.52 ± 1.37 a | 69.24 ± 0.23 b | 83.19 ± 0.61 a |
| Pecan nut shell | 74.79 ± 1.55 b | 90.32 ± 0.33 a | 92.26 ± 0.24 a | 91.66 ± 0.32 a |
| Extract | FW | FE | CW | CE |
|---|---|---|---|---|
| Jaboticaba peel | 1342.95 ± 2.73 b | 3525.69 ± 405.33 a | 1402.32 ± 144.62 b | 2111.17 ± 17.67 b |
| Olive leaf | 623.61 ± 37.01 ab | 484.14 ± 17.97 ab | 451.91 ± 3.72 b | 708.33 ± 71.12 a |
| Araçá peel | 128.21 ± 4.14 c | 222.53 ± 7.29 b | 171.98 ± 4.56 b | 424.19 ± 23.56 a |
| Pecan nut shell | 3449.46 ± 409.27 ab | 4266.53 ± 166.33 a | 2621.29 ± 100.68 b | 3979.07 ± 132.54 ab |
| Extract | FW | FE | CW | CE |
|---|---|---|---|---|
| Jaboticaba peel | 88.02 ± 0.91 b | 122.63 ± 1.79 a | 46.84 ± 1.95 c | 81.47 ± 1.01 b |
| Olive leaf | 41.64 ± 0.65 c | 56.45 ± 0.91 b | 46.31 ± 1.04 c | 67.90 ± 1.42 a |
| Araçá peel | 17.52 ± 0.39 c | 30.61 ± 0.99 a | 13.94 ± 0.15 c | 25.63 ± 0.93 b |
| Pecan nut shell | 154.82 ± 5.67 b | 184.61 ± 1.69 a | 153.61 ± 1.07 b | 180.69 ± 2.56 a |
| Phenolic Compounds | Extracts | |||||||
|---|---|---|---|---|---|---|---|---|
| Jaboticaba Peel | Olive Leaf | Pecan Nut Shell | Araçá Peel | |||||
| Water | Ethanol | Water | Ethanol | Water | Ethanol | Water | Ethanol | |
| Gallic acid | 1.23 ± 0.04 a | 0.32 ± 0.01 d | 0.06 ± 0.00 f | ND | 0.75 ± 0.00 b | 0.22 ± 0.03 de | 0.16 ± 0.01 ef | 0.50 ± 0.04 c |
| Caffeic acid | 0.23 ± 0.02 b | 0.47 ± 0.02 a | 0.07 ± 0.00 c | 0.08 ± 0.00 c | NI | NI | 0.10 ± 0.00 c | 0.20 ± 0.00 b |
| p-Coumaric acid | 0.39 ± 0.01 b | 0.59 ± 0.05 a | 0.22 ± 0.00 c | 0.23 ± 0.00 c | NI | NI | 0.23 ± 0.00 c | 0.24 ± 0.00 c |
| Chlorogenic acid | NI | NI | 0.28 ± 0.00 b | 0.40 ± 0.04 a | NI | NI | 0.20 ± 0.00 b | 0.24 ± 0.01 b |
| trans-Cinnamic acid | NQ | NQ | 0.08 ± 0.00 b | 0.10 ± 0.00 a | NI | NI | 0.04 ± 0.00 c | 0.05 ± 0.00 c |
| trans-Ferulic acid | NQ | NQ | 0.28 ± 0.00 a | 0.22 ± 0.00 b | NI | NI | NQ | NQ |
| Kaempferol | 0.42 ± 0.00 c | 0.43 ± 0.00 c | 0.48 ± 0.00 b | 0.52 ± 0.01 a | NI | NI | NQ | NQ |
| Quercetin | ND | ND | 0.71 ± 0.00 b | 0.75 ± 0.02 a | NI | NI | 0.54 ± 0.00 d | 0.61 ± 0.00 c |
| Cyanidin-3-glucoside | 8.22 ± 0.34 a | 8.83 ± 0.70 a | NI | NI | NI | NI | 1.65 ± 0.00 b | 1.63 ± 0.00 b |
| Hydroxytyrosol | NI | NI | 3.40 ± 0.03 a | 3.71 ± 0.54 a | NI | NI | NI | NI |
| Tyrosol | NI | NI | 1.20 ± 0.00 a | 1.11 ± 0.23 a | NI | NI | NI | NI |
| Oleuropein | NI | NI | 66.81 ± 0.11 b | 130.45 ± 6.07 a | NI | NI | NI | NI |
| Verbascoside | NI | NI | 4.66 ± 0.07 b | 12.42 ± 0.61 a | NI | NI | NI | NI |
| TPI | 10.41 ± 0.41 | 10.64 ± 0.78 | 78.25 ± 0.21 | 149.99 ± 7.52 | 0.75 ± 00 | 0.22 ± 0.03 | 2.92 ± 0.01 | 3.47 ± 0.05 |
| Concentration of Extracts (%, v/v) | ||||||
|---|---|---|---|---|---|---|
| Bacterium | Extracts | 20 | 40 | 60 | 80 | 90 |
| Staphylococcusaureus | Jaboticaba peel | NI | NI | I | I | I |
| Olive leaf | NI | NI | I | I | I | |
| Pecan nut shell | - | - | - | - | - | |
| Araçá peel | NI | NI | NI | NI | I | |
| Escherichiacoli | Jaboticaba peel | NI | NI | I | I | I |
| Olive leaf | NI | NI | I | I | I | |
| Pecan nut shell | - | - | - | - | - | |
| Araçá peel | NI | NI | NI | NI | I | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, A.V.; Avila, L.B.; Lacorte, D.H.; Martiny, T.R.; Rosseto, V.; Moraes, C.C.; Dotto, G.L.; Carreno, N.L.V.; da Rosa, G.S. Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities. Molecules 2022, 27, 6876. https://doi.org/10.3390/molecules27206876
Filho AV, Avila LB, Lacorte DH, Martiny TR, Rosseto V, Moraes CC, Dotto GL, Carreno NLV, da Rosa GS. Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities. Molecules. 2022; 27(20):6876. https://doi.org/10.3390/molecules27206876
Chicago/Turabian StyleFilho, Alaor Valério, Luisa Bataglin Avila, Douglas Hardt Lacorte, Thamiris Renata Martiny, Vanessa Rosseto, Caroline Costa Moraes, Guilherme Luiz Dotto, Neftali Lenin Villarreal Carreno, and Gabriela Silveira da Rosa. 2022. "Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities" Molecules 27, no. 20: 6876. https://doi.org/10.3390/molecules27206876
APA StyleFilho, A. V., Avila, L. B., Lacorte, D. H., Martiny, T. R., Rosseto, V., Moraes, C. C., Dotto, G. L., Carreno, N. L. V., & da Rosa, G. S. (2022). Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities. Molecules, 27(20), 6876. https://doi.org/10.3390/molecules27206876