3.5. Methyl (4-Carbomethoxy-2-fluorophenyl)acetate (12)
To a solution of the 5-cyano-2-fluorobenzaldehyde (11, 10.0 g, 67.1 mmol) in absolute ethanol (80 mL) at 0 °C (ice-water bath), NaBH4 (1.27 g, 33.5 mmol) was added portion-wise with stirring during 10–15 min. The cooling bath was removed and the reaction was allowed to warm to room temperature for 1 h. The reaction was quenched by addition to saturated NaCl (250 mL) and extracted with ether (3 × 50 mL). The combined ether layers were washed with saturated NaCl (3 × 50 mL) and dried (Na2SO4). Filtration and concentration under vacuum gave the alcohols as off-white solids that were purified by trituration with 2% ether in pentane to give (5-cyano-2-fluorophenyl)methanol (9.22 g, 91%) as an off-white solid, m.p. 48–50 °C; IR: 3429, 2234 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.84 (dd, J = 6.7, 2.1 Hz, 1H), 7.61 (ddd, J = 8.3, 4.8, 2.1 Hz, 1H), 7.16 (t, J = 9.0 Hz, 1H), 4.80 (s, 2H), 2.16 (br s, 1H); 13C NMR (101 MHz, CDCl3): δ 162.5 (d, J = 256.6 Hz), 133.5 (d, J = 9.5 Hz), 133.1 (d, J = 6.2 Hz), 130.1 (d, J = 16.1 Hz), 118.4, 116.5 (d, J = 22.9 Hz), 108.6 (d, J = 3.9 Hz), 58.2 (d, J = 4.4 Hz); MS (m/z) 151 (M+); Anal. Calcd for C8H6FNO: C, 63.58; H, 4.00; N, 9.27. Found: C, 63.65; H, 3.97; N, 9.22.
A solution of (5-cyano-2-fluorophenyl)methanol (9.22 g, 61.1 mmol) in anhydrous ether (100 mL) was cooled to 0 °C. To the stirred solution was added drop-wise PBr3 (8.32 g, 2.89 mL, 30.7 mmol) during 1 h. The reaction was allowed to warm to 23 °C overnight at which time it was quenched by addition to saturated NaCl (150 mL). The ether layer was separated and the aqueous layer was washed with ether (2 × 50 mL). The combined ether layers were washed with saturated NaCl (3 × 50 mL) and dried (Na2SO4). Filtration and concentration under vacuum gave the bromide as an off-white solid which was purified by trituration with 2% ether in pentane to give 2-(bromomethyl)-4-cyano-1-fluorobenzene (11.8 g, 90%) as an off-white solid, m.p. 55–56 °C; IR: 2238 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.76 (dd, J = 6.9, 2.2 Hz, 1H), 7.64 (ddd, J = 8.6, 4.8, 2.2 Hz, 1H), 7.20 (t, J = 8.9 Hz, 1H), 4.48 (s, 2H); 13C NMR (101 MHz, CDCl3): δ 162.9 (d, J = 260.0 Hz), 135.5, (d, J = 4.5 Hz), 134.6 (d, J = 9.7 Hz), 127.3 (d, J = 16.0 Hz), 117.5, 117.3 (d, J = 22.9 Hz), 109.1 (d, J = 4.0 Hz), 23.5 (d, J = 4.3 Hz); MS (m/z) 213, 215 (ca 1:1, M+); Anal. Calcd for C8H5FBrN: C, 44.89; H, 2.35; N, 6.54. Found: C, 44.97; H, 2.39; N, 6.44.
To a solution of KCN (5.16 g, 79.2 mmol) in water (6 mL) at 23 °C was added drop-wise a solution of the 2-(bromomethyl)-4-cyano-1-fluorobenzene (11.3 g, 52.8 mmol) in ethanol (75–90 mL) during 1 h. The solution was stirred for 16 h and quenched by addition to saturated NaCl (250 mL) and extracted with ether (3 × 50 mL). The combined ether layers were washed with saturated NaCl (3 × 50 mL) and dried (Na2SO4). Filtration and concentration under vacuum gave 3-(cyanomethyl)-4-fluorobenzonitrile as a light tan solid which was subjected to methanolysis without further purification.
A solution of concentrated sulfuric acid in methanol (100 mL, 25% v/v) was prepared at 0 °C. The benzylic nitrile was added slowly and the mixture was heated to boiling for 16 h (bath temperature 90 °C). After cooling, the reaction was quenched by addition to saturated NaCl (250 mL) and extracted with ether (3 × 50 mL). The combined ether layers were washed with saturated NaCl (3 × 50 mL) and dried (Na2SO4). Filtration and concentration under vacuum gave the ester as a yellow oil. Purification was accomplished by silica gel column chromatography (40 cm × 2.5 cm) eluted with 10–15% ether in hexanes to give 7.05 g (60%, 2 steps) of diester 12 as a light yellow oil. IR: 1735, 1720 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.98 (m, 2H), 7.12 (t, J = 9.1 Hz, 1H), 3.91 (d, J = 2.6 Hz, 2H), 3.73 (s, 3H), 3.72 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 170.6, 166.0, 164.0 (d, J = 254.4 Hz), 133.5 (d, J = 5.4 Hz), 131.1 (d, J = 9.5 Hz), 126.5 (d, J = 3.4 Hz), 121.8 (d, J = 16.9 Hz), 115.6 (d, J = 22.9 Hz), 53.3, 52.2, 34.2 (d, J = 2.7 Hz); MS (m/z) 226 (M+); Anal. Calcd for C11H11FO4: C, 58.41; H, 4.90. Found: C, 58.39; H, 4.94.
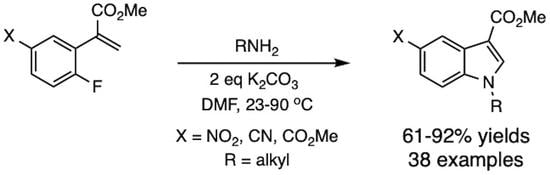
3.6. General Procedure for Conversion of the 2-Arylacetate Esters to Acrylates
The basic procedure of Selvakumar and co-workers was used [
25]. To a mixture of the methyl 2-arylacetate (8.0 mmol) in formalin (37%, 18 mL) was added a suspension of anhydrous K
2CO
3 (1.66 g, 12.0 mmol) in DMF (5 mL). The resulting mixture was heated to 60 °C for 2 h and then cooled to 23 °C. The crude reaction mixture was quenched with water (75 mL) and extracted with ether (3 × 50 mL). The combined ether extracts were washed with saturated NaCl (3 × 50 mL) and dried (Na
2SO
4). Filtration and concentration under vacuum gave the crude product as a light yellow oil. Purification by silica gel column chromatography (25 cm × 2.5 cm) eluted with increasing concentrations of ether in hexanes afforded the pure acrylate esters, which solidified on standing.
3.6.1. Methyl 2-(2-Fluoro-5-nitrophenyl)acrylate (7)
Yield: 1.97 g (60%) as an off-white solid, m.p. 52–54 °C; IR: 1730, 1634, 1527, 1350 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.24 (m, 2H), 7.24 (t, J = 8.8 Hz, 1H), 6.68 (s, 1H), 6.03 (s, 1H), 3.83 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 165.3 163.4 (d, J = 259.7 Hz), 144.1, 134.5, 131.6 (d, J = 1.7 Hz), 126.9 (d, J = 5.3 Hz), 126.4 (d, J = 17.4 Hz), 125.8 (d, J = 10.3 Hz), 116.6 (d, J = 24.9 Hz), 52.7; MS (m/z) 225 (M+); Anal. Calcd for C10H8FNO4: C, 53.34; H, 3.58; N, 6.22. Found: C, 53.37; H, 3.61; N, 6.13.
3.6.2. Methyl 2-(5-Cyano-2-fluorophenyl)acrylate (10)
Yield: 1.66 g (58%) as a yellow solid, m.p. 69–70 °C; IR: 2231, 1723 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.66 (ddd, J = 8.5, 4.7, 2.2 Hz, 1H), 7.63 (dd, J = 6.7, 2.2 Hz, 1H), 7.20 (dd, J = 9.2, 8.5 Hz, 1H), 6.64 (d, J = 0.8 Hz, 1H), 5.97 (d, J = 0.7 Hz, 1H), 3.82 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 165.4, 162.3 (d, J = 258.4 Hz), 135.2 (d, J = 4.5 Hz), 134.5, 134.3 (d, J = 9.8 Hz), 131.3 (d, J = 1.6 Hz), 126.9 (d, J = 16.7 Hz), 117.8, 117.1 (d, J = 23.9 Hz), 108.6 (d, J = 4.0 Hz), 52.6; MS (m/z): 205 (M+); Anal. Calcd for C11H8FNO2: C, 64.39; H, 3.93; N, 6.83. Found: C, 64.33; H, 3.96; N, 6.78.
3.6.3. Methyl 2-(5-Carbomethoxy-2-fluorophenyl)acrylate (13)
Yield: 2.03 g (62%) as an off-white solid, m.p. 52–54 °C; IR: 1724, 1627 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (ddd, J = 8.6, 5.0, 2.3 Hz, 1H), 8.00 (dd, J = 7.1, 2.3 Hz, 1H), 7.13 (t, J = 9.0 Hz, 1H), 6.58 (d, J = 1.1 Hz, 1H), 5.95 (d, J = 1.1 Hz, 1H), 3.92 (s, 3H), 3.81 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 166.0, 165.9, 162.8 (d, J = 256.0 Hz), 135.7, 132.8(d, J = 4.7 Hz), 132.0 (d, J = 9.7 Hz), 130.3, 126.4 (d, J = 3.3 Hz), 125.4 (d, J = 16.0 Hz), 115.7 (d, J = 23.1 Hz), 52.5, 52.3; MS (m/z) 238 (M+); Anal. Calcd for C12H11FO4: C, 60.51; H, 4.65. Found: C, 60.48; H, 4.64.
3.8. Reactions with Methyl 2-(2-Fluoro-5-nitrophenyl)acrylate (7)
3.8.1. Methyl 1-Hexyl-5-nitro-1H-indole-3-carboxylate (17a)
Yield: 243 mg (80%) as a light yellow solid, m.p. 52–54 °C; IR: 1707, 1612, 1537, 1341 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.08 (d, J = 2.3 Hz, 1H), 8.18 (dd, J = 9.1, 2.3 Hz, 1H), 7.97 (s, 1H), 7.41 (d, J = 9.1 Hz, 1H), 4.19 (t, J = 7.1 Hz, 2H), 3.97 (s, 3H), 1.88 (quintet, J = 7.1 Hz, 2H), 1.31 (m, 6H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 164.5, 143.4, 139.2, 136.9, 125.9, 119.0, 118.3, 110.2, 109.5, 51.5, 47.5, 31.2, 29.9, 26.4, 22.4, 13.9; MS (m/z): 304 (M+); Anal. Calcd for C16H20N2O4: C, 63.14; H, 6.62; N, 9.20. Found: C, 63.11; H, 6.59; N, 9.14.
3.8.2. Methyl 1-Isobutyl-5-nitro-1H-indole-3-carboxylate (17b)
Yield: 243 mg (88%) as a light yellow solid, m.p. 131–133 °C; IR: 1706, 1614, 1539, 1347 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.06 (d, J = 2.3 Hz, 1H), 8.16 (dd, J = 9.1, 2.3 Hz, 1H), 7.96 (s, 1H), 7.40 (d, J = 9.1 Hz, 1H), 4.00 (d, J = 7.4 Hz, 2H), 3.96 (s, 3H), 2.21 (nonet, J = 6.9 Hz, 1H), 0.97 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3): δ 164.4, 143.3, 139.5, 137.5, 125.8, 119.8, 118.2, 110.4, 109.3, 55.0, 51.5, 29.5, 20.1; MS (m/z): 276 (M+); Anal. Calcd for C14H16N2O4: C, 60.86; H, 5.84; N, 10.14. Found: C, 60.83; H, 5.82; N, 10.17.
3.8.3. Methyl 1-Allyl-5-nitro-1H-indole-3-carboxylate (17c)
Yield: 234 mg (90%) as a light yellow solid, m.p. 119–121 °C; IR: 1704, 1620, 1534, 1342 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.06 (d, J = 2.3 Hz, 1H), 8.16 (dd, J = 9.1, 2.3 Hz, 1H), 7.96 (s, 1H), 7.40 (d, J = 9.1 Hz, 1H), 6.01 (ddt, J = 17.0, 10.6, 5.4 Hz, 1H), 5.35 (d, J = 10.6 Hz, 1H), 5.18 (d, J = 17.0 Hz, 1H), 4.82 (d, J = 5.4 Hz, 2H), 3.96 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.3, 143.5, 139.3, 137.0, 131.2, 126.0, 119.4, 118.8, 118.4, 110.4, 109.7, 51.5, 49.8; MS (m/z): 260 (M+); Anal. Calcd for C13H12N2O4: C, 60.00; H, 4.65; N, 10.76. Found: C, 59.97; H, 4.67; N, 10.58.
3.8.4. Methyl 1-Cyclopropyl-5-nitro-1H-indole-3-carboxylate (17d)
Yield: 195 mg (75%) as a light yellow solid, m.p. 133–134 °C; IR: 1698, 1607, 1533, 1334 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.03 (d, J = 2.3 Hz, 1H), 8.19 (dd, J = 9.1, 2.3 Hz, 1H), 7.98 (s, 1H), 7.64 (d, J = 9.1 Hz, 1H), 3.95 (s, 3H), 3.48 (septet, J = 3.8 Hz, 1H), 1.24 (m, 2H), 1.08 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 164.3, 143.7, 140.7, 137.3, 125.9, 118.8, 118.4, 111.0, 109.4, 51.5, 28.0, 6.5; MS (m/z): 260 (M+); Anal. Calcd for C13H12N2O4: C, 60.00; H, 4.65; N, 10.76. Found: C, 60.04; H, 4.65; N, 10.61.
3.8.5. Methyl 1-Cyclohexyl-5-nitro-1H-indole-3-carboxylate (17e)
Yield: 236 mg (78%) as a light yellow solid, m.p. 133–134 °C; IR: 1708, 1614, 1532, 1339 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.07 (d, J = 2.3 Hz, 1H), 8.16 (dm, J = 9.1 Hz, 1H), 8.09 (s, 1H), 7.46 (d, J = 9.1 Hz, 1H), 4.28 (tt, J = 12.0, 3.7 Hz, 1H), 3.96 (s, 3H), 2.20 (d, J = 12.0 Hz, 2H), 2.00 (d, J = 13.5, 3.6 Hz, 2H), 1.85 (d, J = 13.1 Hz, 1H), 1.74 (qd, J = 13.1, 3.6 Hz, 2H), 1.55 (qt, J = 13.1, 3.6 Hz, 2H), 1.31 (qt, J = 13.1, 3.6 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 164.6, 143.3, 138.9, 133.8, 125.8, 118.9, 118.0, 110.1, 109.5, 56.4, 51.4, 33.4, 25.7, 25.3; MS (m/z): 302 (M+); Anal. Calcd for C16H18N2O4: C, 63.56; H, 6.00; N, 9.27. Found: C, 63.63; H, 6.05; N, 9.23.
3.8.6. Methyl 1-(tert-Butyl)-5-nitro-1H-indole-3-carboxylate (17f)
Yield: 213 mg (77%) as a light yellow solid, m.p. 161–163 °C; IR: 1708, 1615, 1536, 1342 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.11 (d, J = 2.4 Hz, 1H), 8.15 (s, 1H), 8.14 (dd, J = 9.3, 2.4 Hz, 1H), 7.72 (d, J = 9.3 Hz, 1H), 3.96 (s, 3H), 1.79 (s, 9H); 13C NMR (101 MHz, CDCl3): δ 164.6, 142.8, 138.3, 135.1, 127.6, 118.8, 117.4, 113.8, 108.4, 58.0, 51.4, 29.7; MS (m/z): 276 (M+); Anal. Calcd for C14H16N2O4: C, 60.86 H, 5.84; N, 10.14. Found: C, 60.84; H, 5.81; N, 10.06.
3.8.7. Methyl 5-Nitro-1-phenethyl-1H-indole-3-carboxylate (17g)
Yield: 275 mg (85%) as a light yellow solid, m.p. 165–167 °C; IR: 1705, 1619, 1536, 1341 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.05 (d, J = 2.3 Hz, 1H), 8.11 (dd, J = 9.1, 2.3 Hz, 1H), 7.77 (s, 1H), 7.28–7.22 (complex, 4H), 7.00 (complex, 2H), 4.43 (t, J = 7.0 Hz, 2H), 3.94 (s, 3H), 3.15 (t, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 164.4, 143.4. 139.1, 137.0, 136.9, 129.0, 128.6, 127.3, 125.8, 118.9, 118.3, 110.0, 109.6, 51.5, 49.2, 36.6; MS (m/z): 324 (M+); Anal. Calcd for C18H16N2O4: C, 66.66; H, 4.97; N, 8.64. Found: C, 66.71; H, 4.96; N, 8.57.
3.8.8. Methyl 1-Benzyl-5-nitro-1H-indole-3-carboxylate (17h)
Yield: 263 mg (85%) as a light yellow solid, m.p. 112–113 °C; IR: 1705, 1618, 1537, 1341 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.09 (d, J = 2.2 Hz, 1H), 8.14 (dd, J = 9.1, 2.2 Hz, 1H), 7.98 (s, 1H), 7.39–7.32 (complex, 4H), 7.17–7.14 (complex, 2H), 5.39 (s, 2H), 3.96 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.3, 143.5, 139.4, 137.3, 134.8, 129.3, 128.7, 127.1, 126.1, 118.9, 118.6, 110.6, 110.0, 51.5, 51.3; MS (m/z): 310 (M+); Anal. Calcd for C17H14N2O4: C, 65.80; H, 4.55; N, 9.03. Found: C, 65.74; H, 4.51; N, 8.98.
3.8.9. Methyl 1-(4-Methylbenzyl)-5-nitro-1H-indole-3-carboxylate (17i)
Yield: 279 g (86%) as a light yellow solid, m.p. 163–164 °C; IR: 1706, 1616, 1538, 1349 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.07 (d, J = 2.2 Hz, 1H), 8.13 (dd, J = 9.1, 2.2 Hz, 1H), 7.96 (s, 1H), 7.37 (d, J = 9.1 Hz, 1H), 7.16 (d, J = 7.9 Hz, 2H), 7.06 (d, J = 7.9 Hz, 2H), 5.33 (s, 2H), 3.95 (s, 3H), 2.33 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.4, 143.5, 139.4, 138.6, 137.2, 131.7, 129.9, 127.2, 126.1, 118.9, 118.5, 110.6, 109.8, 51.5, 51.1, 21.1; MS (m/z): 324 (M+); Anal. Calcd for C18H16N2O4: C, 66.66; H, 4.97; N, 8.64. Found: C, 66.61; H, 4.95; N, 8.62.
3.8.10. Methyl 1-(3-Methoxybenzyl)-5-nitro-1H-indole-3-carboxylate (17j)
Yield: 282 mg (83%) as a light yellow solid, m.p. 112–113 °C; IR: 2840, 1704, 1611, 1547, 1338 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.09 (d, J = 2.2 Hz, 1H), 8.14 (dd, J = 9.1, 2.2 Hz, 1H), 7.98 (s, 1H), 7.37 (d, J = 9.1 Hz, 1H), 7.27 (t, J = 7.8 Hz, 1H), 6.87 (dd, J = 8.6, 2.2 Hz, 1H), 6.73 (d, J = 7.8 Hz, 1H), 6.67 (t, J = 2.2 Hz, 1H), 5.35 (s, 2H), 3.96 (s, 3H), 3.76 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.3, 160.2, 143.5, 139.4, 137.3, 136.3, 130.4, 126.1, 119.3, 118.9, 118.6, 113.5, 113.2, 110.6, 110.0, 55.3, 51.5, 51.2; MS (m/z): 340 (M+); Anal. Calcd for C18H16N2O5: C, 63.53; H, 4.74; N, 8.23. Found: C, 63.44; H, 4.77; N, 8.17.
3.8.11. Methyl 1-(4-Methoxybenzyl)-5-nitro-1H-indole-3-carboxylate (17k)
Yield: 296 mg (87%) as a light yellow solid, m.p. 153–154 °C; IR: 2838, 1705, 1614, 1537, 1342 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.08 (d, J = 2.3 Hz, 1H), 8.14 (dd, J = 9.1, 2.3 Hz, 1H), 7.95 (s, 1H), 7.39 (d, J = 9.1 Hz, 1H), 7.12 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.7 Hz, 2H), 5.31 (s, 2H), 3.95 (s, 3H), 3.80 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.4, 159.9, 143.5, 139.3, 137.1, 128.8, 126.6, 126.1, 118.9, 118.5, 114.6, 110.6, 109.8, 55.4, 51.5, 50.8; MS (m/z): 340 (M+); Anal. Calcd for C18H16N2O5: C, 63.53; H, 4.74; N, 8.23. Found: C, 63.49; H, 4.74; N, 8.21.
3.8.12. Methyl 1-(4-Chlorobenzyl)-5-nitro-1H-indole-3-carboxylate (17l)
Yield: 279 mg (81%) as a light yellow solid, m.p. 167–169 °C; IR: 1706, 1611, 1537, 1342 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.07 (d, J = 2.2 Hz, 1H), 8.13 (dd, J = 9.1, 2.2 Hz, 1H), 7.96 (s, 1H), 7.33 (d, J = 8.5 Hz, 2H), 7.32 (d, J = 9.1 Hz, 1H), 7.09 (d, J = 8.5 Hz, 2H), 5.37 (s, 2H), 3.96 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.2, 149.6, 139.2, 137.1, 134.7, 133.3, 129.5, 128.4, 126.1, 119.0, 118.7, 110.5, 110.2, 51.6, 50.6; MS (m/z): 344 (M+); Anal. Calcd for C17H13ClN2O4: C, 59.23; H, 3.80; N, 8.13. Found: C, 59.16; H, 3.77; N, 8.07.
3.8.13. Methyl 5-Nitro-1-((4-trifluoromethyl)benzyl)-1H-indole-3-carboxylate (17m)
Yield: 336 mg (89%) as a light yellow solid, m.p. 162–164 °C; IR: 1707, 1616, 1538, 1343, 1325 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.11 (d, J = 2.3 Hz, 1H), 8.15 (dd, J = 9.1, 2.3 Hz, 1H), 7.99 (s, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.43 (s, 1H), 7.32 (d, J = 9.1 Hz, 1H), 7.24 (dt, J = 8.1 Hz, 1H), 5.47 (s, 2H), 3.97 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.1, 143.7, 139.2, 138.9, 137.1, 131.0 (q, J = 32.8 Hz), 127.2, 126.3 (q, J = 3.7 Hz), 126.1, 123.7 (q, J = 272.4 Hz), 119.1, 118.8, 110.5, 110.4, 51.6, 50.7; MS (m/z): 378 (M+); Anal. Calcd for C18H13F3N2O4: C, 57.15; H, 3.46; N, 7.41. Found: C, 57.24; H, 3.49; N, 7.30.
3.8.14. Methyl 5-Nitro-1-(3-nitrobenzyl)-1H-indole-3-carboxylate (17n)
Yield: 323 mg (91%) as a yellow solid, m.p. 147–149 °C; IR: 1706, 1618, 534, 1344 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.11 (d, J = 2.3 Hz, 1H), 8.24 (d, J = 8.1 Hz, 1H), 8.16 (dd, J = 9.1, 2.3 Hz, 1H), 8.08 (br s, 1H), 8.02 (s, 1H), 7.56 (t, J = 8.0 Hz, 1H), 7.41 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 9.1 Hz, 1H), 5.52 (s, 2H), 3.98 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.1, 148.8, 143.8, 139.1, 137.1, 136.9, 132.6, 130.5, 126.2, 123.7, 121.9, 119.2, 119.0, 110.8, 110.2, 51.7, 50.4; MS (m/z): 355 (M+); Anal. Calcd for C17H13N3O6: C, 57.47; H, 3.69; N, 11.83. Found: C, 57.39; H, 3.65; N, 11.72.
3.8.15. Methyl 1-Amino-5-nitro-1H-indole-3-carboxylate (17o)
Yield: 195 mg (83%) as a tan solid, m.p. 199–200 °C; IR: 3333, 3125, 1698 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.86 (d, J = 2.3 Hz, 1H), 8.19 (s, 1H), 8.18 (dd, J = 9.1, 2.3 Hz, 1H), 7.75 (d, J = 9.1 Hz, 1H), 6.49 (s, 2H), 3.86 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 163.0, 142.0, 139.2, 138.5, 122.5, 117.1, 116.4, 111.1, 104.3, 50.6; MS (m/z): 235 (M+); Anal. Calcd for C10H9N3O4: C, 51.07; H, 3.86; N, 17.87. Found: C, 51.08; H, 3.83; N, 17.77.
3.9. Reactions with Methyl 2-(5-Cyano-2-fluorophenyl)acrylate (10)
3.9.1. Methyl 5-cyano-1-hexyl-1H-indole-3-carboxylate (18a)
Yield: 210 mg (74%) as a white solid, m.p. 71–73 °C; IR: 2223, 1705, 1612 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.53 (br s, 1H), 7.92 (s, 1H), 7.51 (dd, J = 8.6, 1.6 Hz, 1H), 7.42 (dd, J = 8.6, 0.8 Hz, 1H), 4.15 (t, J = 7.1 Hz, 2H), 3.94 (s, 3H), 1.88 (quintet, J = 7.1 Hz, 2H), 1.31 (m, 6H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 164.6, 138.0, 136.1, 127.4, 126.3, 125.6, 120.2, 111.0, 108.0, 105.1, 51.4, 47.3, 31.2, 29.7, 26.4, 22.4, 14.1; MS (m/z): 284 (M+); Anal. Calcd for C17H20N2O2: C, 71.81; H, 7.09; N, 9.85. Found: C, 71.87; H, 7.13; N, 9.73.
3.9.2. Methyl 5-Cyano-1-isobutyl-1H-indole-3-carboxylate (18b)
Yield: 172 mg (67%) as a white solid, m.p. 70–72 °C; IR: 2223, 1705, 1612 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.53 (br s, 1H), 7.90 (s, 1H), 7.50 (dd, J = 8.6, 1.6 Hz, 1H), 7.42 (dd, J = 8.6, 0.7 Hz, 1H), 3.98 (d, J = 7.4 Hz, 2H), 3.94 (s, 3H), 2.21 (nonet, J = 6.9 Hz, 1H), 0.95 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3): δ 164.6, 138.3, 136.6, 127.4, 126.3, 125.6, 120.2, 111.2, 108.0, 105.1, 54.8, 51.4, 29.4, 20.1; MS (m/z) 256 (M+); Anal. Calcd for C15H16N2O2: C, 70.29; H, 6.29; N, 10.93. Found: C, 70.26; H, 6.27; N, 10.87.
3.9.3. Methyl 5-Cyano-1-cyclopropyl-1H-indole-3-carboxylate (18d)
Yield: 187 mg (78%) as a white solid, m.p. 132–134 °C; IR: 2222, 1706, 1614 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.48 (br s, 1H), 7.94 (s, 1H), 7.65 (dd, J = 8.5, 0.8 Hz, 1H), 7.51 (dd, J = 8.5, 1.6 Hz, 1H), 3.92 (s, 3H), 3.45 (apparent septet, J = 3.6 Hz, 1H), 1.21 (m, 2H), 1.06 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 164.4, 139.5, 136.4, 127.3, 126.4, 125.8, 120.2, 111.8, 108.0, 105.5, 51.4, 27.8, 6.4; MS (m/z): 240 (M+); Anal. Calcd for C14H12N2O2: C, 69.99; H, 5.03; N, 11.66. Found: C, 69.94; H, 4.97; N, 11.59.
3.9.4. Methyl 5-Cyano-1-cyclohexyl-1H-indole-3-carboxylate (18e)
Yield: 223 mg (79%) as a white solid, m.p. 132–134 °C; IR: 2223, 1705, 1614 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.53 (br s, 1H), 8.05 (s, 1H), 7.50 (dd, J = 8.6, 1.6 Hz, 1H), 7.47 (dd, J = 8.6, 0.8 Hz, 1H), 4.25 (tt, J = 11.9, 3.7 Hz, 1H), 3.94 (s, 3H), 2.17 (d, J = 12.8 Hz, 2H), 2.00 (dt, J = 13.5, 3.5 Hz, 2H), 1.84 (dd, J = 13.2 Hz, 1H), 1.76 (qd, J = 13.2, 3.5 Hz, 2H), 1.53 (qt, J = 13.2, 3.5 Hz, 2H), 1.33 (qt, J = 13.0, 3.5 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 164.7, 137.7, 132.9, 127.4, 126.3, 125.4, 120.3, 111.0, 108.1, 105.1, 56.1, 51.3, 33.4, 25.7, 25.3; MS (m/z): 282 (M+); Anal. Calcd for C17H18N2O2: C, 72.32; H, 6.43; N, 9.92. Found: C, 72.34; H, 6.44; N, 9.85.
3.9.5. Methyl 5-Cyano-1-phenethyl-1H-indole-3-carboxylate (18g)
Yield: 277 mg (91 %) as a white solid, m.p. 112–114 °C; IR: 2222, 1702, 1615 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.51 (s, 1H), 7.73 (s, 1H), 7.45 (d, J = 8.5 Hz, 1H), 7.30 (d, J = 8.5 Hz, 1H), 7.27–7.20 (complex, 3H), 7.04–6.96 (complex, 2H), 4.42 (t, J = 7.0 Hz, 2H), 3.91 (s, 3H), 3.13 (t, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 164.5, 137.9, 137.1, 136.1, 128.9, 128.6, 127.4, 127.3, 126.2, 125.7, 120.2, 110.9, 108.1, 105.1, 51.4, 48.9, 36.5; MS (m/z): 304 (M+); Anal. Calcd for C19H16N2O2: C, 74.98; H, 5.30; N, 9.20. Found: C, 74.95; H, 5.28; N, 9.14.
3.9.6. Methyl 1-Benzyl-5-cyano-1H-indole-3-carboxylate (18h)
Yield: 255 mg (88%) as a white solid, m.p. 131–133 °C; IR: 2223, 1705, 1614 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.55 (d, J = 1.5 Hz, 1H), 7.94 (s, 1H), 7.47 (dd, J = 8.5, 1.5 Hz, 1H), 7.40–7.32 (complex, 4H), 7.15–7.13 (complex, 2H), 5.36 (s, 2H), 3.93 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.5, 138.2, 136.4, 134.9, 129.2, 128.6, 127.5, 127.1, 126.5, 125.9, 120.1, 111.3, 108.6, 105.4, 51.4, 51.1; MS (m/z): 290 (M+); Anal. Calcd for C18H14N2O2: C, 74.47; H, 4.86; N, 9.65. Found: C, 74.44; H, 4.86; N, 9.63.
3.9.7. Methyl 5-Cyano-1-(4-methylbenzyl)-1H-indole-3-carboxylate (18i)
Yield: 246 mg (81%) as a white solid, m.p. 139–141 °C; IR: 2222, 1704, 1615 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.54 (br s, 1H), 7.92 (s, 1H), 7.46 (dd, J = 8.5, 1.6 Hz, 1H), 7.38 (dd, J = 8.5, 0.8 Hz, 1H), 7.15 (d, J = 7.8 Hz, 2H), 7.05 (d, J = 7.8 Hz, 2H), 5.31 (s, 2H), 3.93 (s, 3H), 2.33 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.5, 138.5, 138.2, 136.4, 131.8, 129.9, 127.4, 127.2, 126.5, 125.9, 120.1, 111.4, 108.4, 105.4, 51.4, 50.9, 21.1; MS (m/z): 304 (M+); Anal. Calcd for C19H16N2O2: C, 74.98; H, 5.30; N, 9.20. Found: C, 74.94; H, 5.29; N, 9.16.
3.9.8. Methyl 5-Cyano-1-(3-methoxybenzyl)-1H-indole-3-carboxylate (18j)
Yield: 265 mg (83%) as a white solid, m.p. 118–120 °C; IR: 2837, 2222, 1702, 1612 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.55 (br s, 1H), 7.94 (s, 1H), 7.47 (dd, J = 8.6, 1.6 Hz, 1H), 7.37 (dd, J = 8.6, 0.7 Hz, 1H), 7.27 (t, J = 8.1 Hz, 1H), 6.86 (dd, J = 8.1, 2.5 Hz, 1H), 6.71 (d, J = 7.6 Hz, 1H), 6.65 (br t, J = 2.1 Hz, 1H), 5.32 (s, 2H), 3.94 (s, 3H), 3.75 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.5, 160.2, 138.2, 136.4, 130.4, 127.5, 126.5, 126.0, 120.1, 119.2, 113.5, 113.2, 111.3, 108.6, 105.5, 55.3, 51.4, 51.0 (one aromatic carbon unresolved); MS (m/z): 320 (M+); Anal. Calcd for C19H16N2O3: C, 71.24; H, 5.03; N, 8.74. Found: C, 71.17; H, 4.99; N, 8.68.
3.9.9. Methyl 5-Cyano-1-(4-methoxybenzyl)-1H-indole-3-carboxylate (18k)
Yield: 285 mg (89%) as a white solid, m.p. 130–132 °C; IR: 2840, 2222, 1703, 1613 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.54 (br s, 1H), 7.90 (s, 1H), 7.47 (dd, J = 8.6, 1.6 Hz, 1H), 7.40 (dd, J = 8.6, 0.7 Hz, 1H), 7.11 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.7 Hz, 2H), 5.28 (s, 2H), 3.93 (s, 3H), 3.79 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.5, 159.8, 138.1, 136.2, 128.7, 127.4, 126.7, 126.5, 125.8, 120.1, 114.6, 111.3, 108.4, 105.4, 55.3, 51.4, 50.6; MS (m/z): 320; (M+) Anal. Calcd for C19H16N2O3: C, 71.24; H, 5.03; N, 8.74. Found: C, 71.23; H, 5.01; N, 8.66.
3.9.10. Methyl 1-(4-Chlorobenzyl)-5-cyano-1H-indole-3-carboxylate (18l)
Yield: 282 mg (87%) as a white solid, m.p. 193–195 °C; IR: 2223, 1704, 1614 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.54 (br s, 1H), 7.93 (s, 1H), 7.46 (dd, J = 8.6, 1.6 Hz, 1H), 7.34 (dd, J = 8.6, 0.7 Hz, 1H), 7.31 (d, J = 8.5 Hz, 2H), 7.08 (d, J = 8.5 Hz, 2H), 5.34 (s, 2H), 3.93 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.3, 138.0, 136.2, 134.6, 133.4, 129.5, 128.4, 127.5, 126.5, 126.0, 120.0, 111.2, 108.8, 105.6, 51.5, 50.4; MS (m/z): 324 (M+); Anal. Calcd for C18H13ClN2O2: C, 66.57; H, 4.03; N, 8.63. Found: C, 66.57; H, 4.02; N, 8.59.
3.9.11. Methyl 5-Cyano-1-(3-nitrobenzyl)-1H-indole-3-carboxylate (18n)
Yield: 308 mg (92%) as a light yellow solid, m.p. 165–167 °C; IR: 2223, 1702, 1614, 1533, 1350 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.57 (br s, 1H), 8.21 (dd, J = 8.2, 1.3 Hz, 1H), 8.07 (br t, J = 2.1 Hz, 1H), 7.98 (s, 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.49 (dd, J = 8.6, 1.6 Hz, 1H), 7.39 (d, J = 7.7 Hz, 1H), 7.33 (d, J = 8.6 Hz, 1H), 5.49 (s, 2H), 3.95 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.2, 148.8, 137.9, 137.2, 136.1, 132.6, 130.5, 127.8, 126.6, 126.4, 123.7, 121.9, 119.8, 111.0, 109.5, 106.0, 51.6, 50.2; MS (m/z): 335 (M+); Anal. Calcd for C18H13N3O4: C, 64.48; H, 3.91; N, 12.53. Found: C, 64.42; H, 3.88; N, 12.47.
3.10. Methyl 2-(5-Carbomethoxy-2-fluorophenyl)acrylate (13)
3.10.1. Dimethyl 1-Hexyl-1H-indole-3,5-dicarboxylate (19a)
Yield: 212 mg (67%) as a white solid, m.p. 64–66 °C; IR: 1719, 1634 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.88 (d, J = 1.7 Hz, 1H), 7.98 (dd, J = 8.7, 1.7 Hz, 1H), 7.87 (s, 1H), 7.37 (d, J = 8.7 Hz, 1H), 4.14 (t, J = 7.2 Hz, 2H), 3.95 (s, 3H), 3.94 (s, 3H), 1.86 (quintet, J = 7.2 Hz, 2H), 1.31 (m, 6H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 167.9, 165.1, 139.0, 135.6, 126.1, 124.5, 124.1, 123.8, 109.8, 108.3, 52.0, 51.2, 47.2, 31.3, 29.9, 26.5, 22.5, 14.0; MS (m/z): 317 (M+); Anal. Calcd for C18H23NO4: C, 68.12; H, 7.30; N, 4.41. Found: C, 68.19; H, 7.31; N, 4.33.
3.10.2. Dimethyl 1-Isobutyl-1H-indole-3,5-dicarboxylate (19b)
Yield: 199 mg (69%) as a white solid, m.p. 125–126 °C; IR: 1717, 1631 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.88 (dd, J = 1.7, 0.7 Hz, 1H), 7.98 (dd, J = 8.7, 1.7 Hz, 1H), 7.86 (s, 1H), 7.37 (dd, J = 8.7, 0.7 Hz, 1H), 3.96 (d, J = 7.2 Hz, 2H), 3.952 (s, 3H), 3.948 (s, 3H), 2.22 (septet, J = 6.8 Hz, 1H), 0.95 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3): δ 167.9, 165.1, 139.2, 136.1, 126.0, 124.5, 124.1, 123.9, 110.0, 108.3, 54.8, 52.0, 51.2, 29.3, 20.2; MS (m/z): 289 (M+); Anal. Calcd for C16H19NO4: C, 66.42; H, 6.62; N, 4.84. Found: C, 66.47; H, 6.65; N, 4.78.
3.10.3. Dimethyl 1-Allyl-1H-indole-3,5-dicarboxylate (19c)
Yield: 218 mg (80 %) as a white solid, m.p. 102–103 °C; IR: 1710, 1645, 1617 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.88 (d, J = 1.7 Hz, 1H), 7.98 (dd, J = 8.7, 1.7 Hz, 1H), 7.88 (s, 1H), 7.36 (d, J = 8.7 Hz, 1H), 6.00 (ddt, J = 15.8, 10.3, 5.4 Hz, 1H), 5.60 (d, J = 10.3 Hz, 1H), 5.35 (d, J = 15.8 Hz, 1H), 4.78 (d, J = 5.4 Hz, 2H), 3.95 (s, 3H), 3.94 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.9, 165.0, 139.0, 135.6, 131.7, 126.1, 124.5, 124.2, 124.0, 118.9, 110.0, 108.7, 52.0, 51.3, 49.5; MS (m/z): 273 (M+); Anal. Calcd for C15H15NO4: C, 65.92; H, 5.53; N, 5.13. Found: C, 65.87; H, 5.48; N, 5.09.
3.10.4. Dimethyl 1-Cyclopropyl-1H-indole-3,5-dicarboxylate (19d)
Yield: 166 mg (61%) as a white solid, m.p. 136–137 °C; IR: 1709, 1621 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.84 (d, J = 1.7 Hz, 1H), 8.00 (dd, J = 8.7, 1.7 Hz, 1H), 7.90 (s, 1H), 7.60 (d, J = 8.7 Hz, 1H), 3.95 (s, 3H), 3.93 (s, 3H), 3.44 (m, 1H), 1.17 (m, 2H), 1.05 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 167.9, 165.0, 140.4, 135.9, 126.1, 124.4, 124.3, 124.2, 110.6, 108.4, 52.0, 51.2, 27.8, 6.3; MS (m/z): 273 (M+); Anal. Calcd for C15H15NO4: C, 65.92; H, 5.53; N, 5.13. Found: C, 65.81; H, 5.55; N, 5.06.
3.10.5. Dimethyl 1-Cyclohexyl-1H-indole-3,5-dicarboxylate (19e)
Yield: 205 mg (65%) as a white solid, m.p. 138–140 °C; IR: 1703, 1618 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.88 (d, J = 1.7 Hz, 1H), 8.01 (s, 1H), 7.98 (dd, J = 8.7, 1.7 Hz, 1H), 7.42 (d, J = 8.7 Hz, 1H), 4.26 (tt, J = 11.9, 3.7 Hz, 1H), 3.95 (s, 3H), 3.94 (s, 3H), 2.18 (d, J = 12.8 Hz, 2H), 1.98 (dd, J = 13.5, 3.5 Hz, 2H), 1.83 (d, J = 13.2 Hz, 1H), 1.74 (qd, J = 13.2, 3.5 Hz, 2H), 1.53 (qt, J = 13.2, 3.5 Hz, 2H), 1.33 (tt, J = 13.0, 3.5 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 167.9, 165.2, 138.6, 132.4, 126.0, 124.5, 123.88, 123.86, 109.8, 108.4, 54.9, 52.0, 51.2, 33.4, 25.7, 25.4; MS (m/z): 315 (M+); Anal. Calcd for C18H21NO4: C, 68.55; H, 6.71; N, 4.44. Found: C, 68.61; H, 6.73; N, 4.42.
3.10.6. Dimethyl 1-Phenethyl-1H-indole-3,5-dicarboxylate (19g)
Yield: 300 mg (89 %) as a white solid, m.p. 152–154 °C; IR: 1706 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.87 (d, J = 1.7 Hz, 1H), 7.96 (dd, J = 8.7, 1.7 Hz, 1H), 7.70 (s, 1H), 7.30 (d, J = 8.7 Hz, 1H), 7.28–7.21 (complex, 3H), 7.04–7.01 (complex, 2H), 4.39 (t, J = 7.2 Hz, 2H), 3.95 (s, 3H), 3.92 (s, 3H), 3.14 (t, J = 7.2 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 167.9, 165.0, 138.8, 137.3, 135.6, 128.8, 128.6, 127.1, 126.1, 124.5, 124.2, 123.9, 109.7, 108.4, 52.0, 51.2, 48.8, 36.5; MS (m/z): 337 (M+); Anal. Calcd for C20H19NO4: C, 71.20; H, 5.68; N, 4.15. Found: C, 71.18; H, 5.68; N, 4.10.
3.10.7. Dimethyl 1-Benzyl-1H-indole-3,5-dicarboxylate (19h)
Yield: 278 mg (86%) as a white solid, m.p. 140–142 °C; IR: 1709, 1611 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.90 (d, J = 1.7 Hz, 1H), 7.94 (dd, J = 8.7, 1.7 Hz, 1H), 7.89 (s, 1H), 7.37–7.30 (complex, 4H), 7.17–7.13 (complex, 2H), 5.35 (s, 2H), 3.942 (s, 3H), 3.937 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.8, 165.0, 139.2, 135.9, 135.4, 129.1, 128.4, 127.1, 126.2, 124.5, 124.4, 124.1, 110.1, 109.0, 52.0, 51.3, 50.9; MS (m/z): 323 (M+); Anal. Calcd for C19H17NO4: C, 70.58; H, 5.30; N, 4.33. Found: C, 70.56; H, 5.27; N, 4.25.
3.10.8. Dimethyl 1-(4-Methylbenzyl)-1H-indole-3,5-dicarboxylate (19i)
Yield: 276 mg (82%) as a white solid, m.p. 146–147 °C; IR: 1710, 1609 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.89 (d, J = 1.7 Hz, 1H), 7.95 (dd, J = 8.7, 1.7 Hz, 1H), 7.87 (s, 1H), 7.34 (d, J = 8.7 Hz, 1H), 7.14 (d, J = 7.9 Hz, 2H), 7.05 (d, J = 7.9 Hz, 2H), 5.29 (s, 2H), 3.94 (s, 3H), 3.93 (s, 3H), 2.32 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.8, 165.0, 139.1, 138.3, 135.8, 132.3, 129.8, 127.2, 126.2, 124.5, 124.3, 124.1, 110.1, 108.8, 52.0, 51.2, 50.7, 21.1; MS (m/z): 337 (M+); Anal. Calcd for C20H19NO4: C, 71.20; H, 5.68; N, 4.15. Found: C, 71.14; H, 5.64; N, 4.11.
3.10.9. Dimethyl 1-(3-Methoxybenzyl)-1H-indole-3,5-dicarboxylate (19j)
Yield: 303 mg (86%) as a white solid, m.p. 131–132 °C; IR: 2831, 1705, 1622 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.89 (br s, 1H), 7.96 (dd, J = 8.7, 1.7 Hz, 1H), 7.89 (s, 1H), 7.34 (d, J = 8.7 Hz, 1H), 7.25 (t, J = 8.1 Hz, 1H), 6.84 (t, J = 2.5 Hz, 1H), 6.74 (d, J = 7.5 Hz, 1H), 6.66 (t, J = 2.5 Hz, 1H), 5.32 (s, 2H), 3.945 (s, 3H), 3.941 (s, 3H), 3.74 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.8, 165.0, 160.1, 139.2, 136.9, 135.9, 130.2, 126.2, 124.5, 124.4, 124.2, 119.3, 113.4, 113.0, 110.1, 109.0, 55.3, 52.0, 51.3, 50.9; MS (m/z): 353 (M+); Anal. Calcd for C20H19NO5: C, 67.98; H, 5.42; N, 3.96. Found: C, 67.91; H, 5.40, N, 3.88.
3.10.10. Dimethyl 1-(4-Methoxybenzyl)-1H-indole-3,5-dicarboxylate (19k)
Yield: 304 mg (86%) as a white solid, m.p. 135–136 °C; IR: 2833, 1705, 1620 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.89 (d, J = 1.7 Hz, 1H), 7.96 (dd, J = 8.7, 1.7 Hz, 1H), 7.86 (s, 1H), 7.36 (d, J = 8.7 Hz, 1H), 7.12 (d, J = 8.6 Hz, 2H), 6.87 (d, J = 8.6 Hz, 2H), 5.28 (s, 2H), 3.94 (s, 3H), 3.93 (s, 3H), 3.79 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.8, 165.0, 159.7, 139.1, 135.7, 128.7, 127.2, 126.3, 124.5, 124.3, 124.1, 114.5, 110.1, 108.8, 55.3, 52.0, 51.3, 50.5; MS (m/z): 353 (M+); Anal. Calcd for C20H19NO5: C, 67.98; H, 5.42; N, 3.96. Found: C, 68.02; H, 5.41; N, 3.93.
3.10.11. Dimethyl 1-(4-Chlorobenzyl)-1H-indole-3,5-dicarboxylate (19l)
Yield: 286 mg (80%) as a white solid, m.p. 140–142 °C; IR: 1709, 1617 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.90 (d, J = 1.7 Hz, 1H), 7.96 (dd, J = 8.7, 1.7 Hz, 1H), 7.88 (s, 1H), 7.31 (d, J = 8.5 Hz, 2H), 7.29 (d, J = 8.7 Hz, 1H), 7.08 (d, J = 8.5 Hz, 2H), 5.32 (s, 2H), 3.946 (s, 3H), 3.943 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.7, 164.9, 139.0, 135.6, 134.4, 133.9, 129.3, 128.4, 126.2, 124.6, 124.5, 124.3, 110.0, 109.2, 52.0, 51.3, 50.3; MS (m/z): 357 (M+); Anal. Calcd for C19H16ClNO4: C, 63.78; H, 4.51; N, 3.91. Found: C, 63.72; H, 4.47; N, 3.83.
3.10.12. Dimethyl 1-(3-Nitrobenzyl)-1H-indole-3,5-dicarboxylate (19n)
Yield: 334 mg (91%) as a white solid, m.p. 234–236 °C; IR: 1695, 1615, 1514, 1330 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.92 (d, J = 1.7 Hz, 1H), 8.19 (d, J = 8.1 Hz, 1H), 8.08 (s, 1H), 7.97 (dd, J = 8.7, 17 Hz, 1H), 7.93 (s, 1H), 7.52 (t, J = 8.0 Hz, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.28 (d, J = 8.7 Hz, 1H), 5.48 (s, 2H), 3.96 (s, 3H), 3.95 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 167.6, 164.7, 148.7, 138.9, 137.7, 135.5, 132.6, 130.4, 126.3, 124.84, 124.80, 124.6, 123.5, 121.9, 109.9, 109.8, 52.1, 51.4, 50.1; MS (m/z): 368 (M+); Anal. Calcd for C19H16N2O6: C, 61.96; H, 4.38; N, 7.61. Found: C, 61.93; H, 4.39; N, 7.57.