New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater
Abstract
:1. Introduction
2. Results
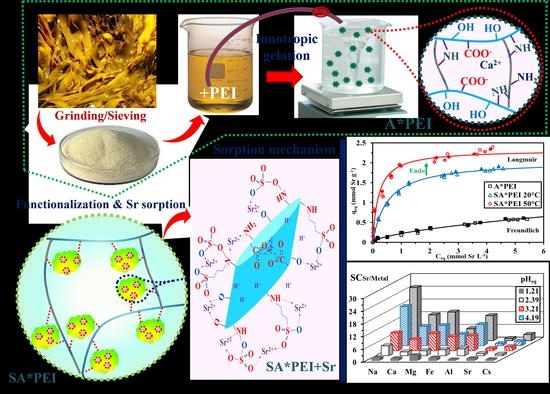
2.1. Characterization of Materials
2.2. Sorption Properties—Synthetic Solutions
2.2.1. pH Effect on Sr(II) Sorption
2.2.2. Uptake Kinetics
2.2.3. Sorption Isotherms
2.2.4. Binding Mechanisms
2.2.5. Sorption Selectivity
2.2.6. Effect of Salinity (NaCl) on Sr(II) Sorption
2.2.7. Sr(II) Desorption from Metal-Loaded SA*PEI and Sorbent Recycling
2.3. Application to Complex Solution—Seawater
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Sorbents
3.3. Characterization of Sorbents
3.4. Sorption and Desorption Procedures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A. Characterization of Materials
Appendix A.1. SEM and SEM-EDX Characterizations


Appendix A.2. Textural Properties

Appendix A.3. Thermal Degradation Properties

Appendix A.4. FTIR Spectroscopy
- 3420 cm−1: ν(N-H) primary amine
- 3280 cm−1: ν(N-H), primary amine and Amide A band
- 1648 cm−1: ν(C=O)/ν(C-N), Amide I band
- 1551 cm−1: ν(N-H)primary in-plane δ(N-H)/ν(C-N)/ν(C-C), Amide II band
- 1241 cm−1: ν(C-N)/δ(N-H), Amide III band
- 1074 cm−1: ν(C-N)

| Vibration | A*PEI | A*PEI + Sr(II) | A*PEI after 5th Desorption | SA*PEI | SA*PEI + Sr(II) | SA*PEI after 5th Desorption | Ref. |
|---|---|---|---|---|---|---|---|
| ν(N-H) + ν(O-H) | 3429 | 3449–3428 | 3447–3429 | 3418 | 3443–3429 | 3422 | [93] |
| ν(C-H) (aliphatic) | 2934 | 2939 | 2934 | 2947 | 2932 | 2953 | [93] |
| ν(C=O) | 1767 | 1721 | 1765 | [93] | |||
| δ(N-H)prim. | 1622 | 1634 | 1620 | 1632 | 1618 | 1622 | [93] |
| O-H, δ(N-H)2nd, δ(C-H) | 1383 | 1429 | 1422 | 1435 | 1429 | 1435 | [93] |
| ν(S=O) | 1339–1315 | ||||||
| ν(C-O) | 1256 | 1256 | |||||
| νs(C-N), νas(S=O) | 1159 | 1171 | 1165 | [94,95] | |||
| ν(C-O-C) carbohydr. | 1092 | 1083 | 1086 | 1084 | 1086 | 1084 | [96] |
| δ(O-H), νs(S=O), νsk(C-O), ν(C-N) | 1034 | 1031 | 1031 | 1030 | 1032 | 1030 | [95,96,97,98] |
| δ(O-H) | 943 | 945 | 941 | 941 | [93] | ||
| ν(S=O) and δ(C-H) | 847, 876 | 818 | 816, 852, 876 | 818, 852 | 816, 851, 878 | 818, 854 | [98,99] |
| Sr-N, Sr-O bonds, ν(S=O) | 602 | 573 | 555 | [99,100] |
- the region 1750–1700 cm−1, assigned to ν(C=O) for carboxylic groups: Shift toward lower wavenumber and formation of a triplet of bands,
- the region 1660–1580 cm−1, assigned to amide bands (overlapped with amine groups): Stronger signal with reduced width,
- the intense and broad band, resulting from the overlapping of different vibrations (O-H, δ(N-H)2nd, δ(C-H)): Shift toward higher wavenumber and width reduction, and
- the region 950–800 cm−1, assigned to δ(O-H), δ(C-H) signals: Variations in the intensity of the relevant signal.

- (a)
- the region at ≈1702 cm−1 (weak shoulder), associated with residual ν(C=O) of carboxylic groups, that disappears (or is shifted toward lower wavenumber around 1652 cm−1, where it contributed to the widening of the band at 1632 cm−1,
- (b)
- the region at ≈1632 cm−1, attributed to δ(N-H) (associated with amide I band): Widening,
- (c)
- the region at ≈1256 cm−1, assigned to ν(C-O): Intensity reduction,
- (d)
- the band at 1159 cm−1, corresponding mainly to νas(S=O): Shift toward higher wavenumber,
- (e)
- the band at 602 cm−1, assigned to ν(S=O): Shifted toward lower wavenumber (and/or replace with a signal associated with Sr-N and Sr-O bond).
- (a)
- the band at 1620 cm−1: Width reduction,
- (b)
- the band at 1383 cm−1: Width reduction and shift toward higher wavenumber, and
- (c)
- the region at 950–800 cm−1: (weak) intensity reductions and shifts of local peaks.
- (a)
- the shoulder at 1702 cm−1: Significantly reduced,
- (b)
- the band at 1159 cm−1: Shift toward higher wavenumber (though less than after Sr(II) sorption, and
- (c)
- the band at 602 cm−1 is again shifted toward the lower wavenumber (more extensive than after Sr(II) sorption).


Appendix A.5. Elemental Analysis and pHPZC
| Sorbent | C (%) | H (%) | O (%) | O (mmol g−1) | N (%) | N (mmol g−1) | S (%) | S (mmol g−1) |
|---|---|---|---|---|---|---|---|---|
| A*PEI | 35.97 | 11.95 | 36.13 | 22.58 | 3.02 | 2.156 | 0.19 | 0.0593 |
| SA*PEI | 34.84 | 13.84 | 43.61 | 27.26 | 2.97 | 2.121 | 3.32 | 1.036 |

Appendix B. Sorption Properties
Appendix B.1. Modeling of Uptake Kinetics and Sorption Isotherms
| (a) | |||
|---|---|---|---|
| Model | Equation | Parameters | Ref. |
| PFORE | qeq,1 (mmol g−1): Sorption capacity at equilibrium k1 (min−1): Apparent rate constant of PFORE | [101] | |
| PSORE | qeq,2 (mmol g−1): Sorption capacity at equilibrium k2 (g mmol−1 min−1): Apparent rate constant of PSORE | [101] | |
| RIDE | With qn being the non-zero roots of and | De (m2 min−1): Effective diffusivity coefficient | [102] |
| (b) | |||
| Model | Equation | Parameters | Ref. |
| Langmuir | qm,L (mmol g−1): Sorption capacity at saturation of monolayer bL (L mmol−1): Affinity coefficient | [103] | |
| Freundlich | kF (mmol g−1)/(mmol L−1)nF and nF: Empirical parameters of Freundlich equation | [104] | |
| Sips | qm,L (mmol g−1), bS (mmol L−1)nS, and nS: Empirical parameters of Sips equation (based on Langmuir and Freundlich equations) | [105] | |
| Temkin | AT (L mmol−1): equilibrium binding capacity; bT: Temkin constant related to sorption heat (J kg−1 mol−2) | [71,106] | |
Appendix B.2. Effect of pH on Sr(II) Sorption



Appendix B.3. Uptake Kinetics
| Sorbent | A*PEI | SA*PEI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | T: 20 ± 1 °C | T: 20 ± 1 °C | T: 50 ± 1 °C | |||||||
| Model | Parameter | # 1 | # 2 | # 3 | # 1 | # 2 | # 3 | # 1 | # 2 | # 3 |
| Experim. | qeq | 0.181 | 0.177 | 0.180 | 0.939 | 0.921 | 0.917 | 1.18 | 1.20 | 1.13 |
| PFORE | qeq,1 | 0.194 | 0.200 | 0.199 | 0.986 | 0.968 | 0.967 | 1.24 | 1.26 | 1.18 |
| k1 × 102 | 2.20 | 1.89 | 2.06 | 5.14 | 5.21 | 4.70 | 5.72 | 5.88 | 5.80 | |
| R2 | 0.976 | 0.949 | 0.971 | 0.975 | 0.974 | 0.973 | 0.977 | 0.976 | 0.977 | |
| AIC | −151 | −137 | −149 | −97 | −97 | −96 | −92 | −92 | −92 | |
| PSORE | qeq,2 | 0.257 | 0.277 | 0.268 | 1.16 | 1.14 | 1.15 | 1.44 | 1.46 | 1.38 |
| k2 × 102 | 7.43 | 5.40 | 6.45 | 5.03 | 5.22 | 4.56 | 4.72 | 4.83 | 5.01 | |
| R2 | 0.962 | 0.929 | 0.955 | 0.936 | 0.935 | 0.937 | 0.937 | 0.937 | 0.937 | |
| AIC | −146 | −134 | −145 | −86 | −86 | −86 | −80 | −80 | −80 | |
| RIDE | De × 109 | 7.06 | 6.54 | 6.93 | 8.30 | 8.54 | 7.48 | 7.22 | 7.14 | 7.26 |
| R2 | 0.956 | 0.920 | 0.947 | 0.939 | 0.939 | 0.938 | 0.936 | 0.935 | 0.936 | |
| AIC | −140 | −129 | −138 | −81 | −82 | −81 | −75 | −74 | −75 | |
Appendix B.4. Sorption Isotherms
| (a) | ||||
|---|---|---|---|---|
| Series # | ||||
| Model | Parameter | 1 | 2 | 3 |
| Experim. | qm,exp. | 0.584 | 0.570 | 0.607 |
| Langmuir | qeq,L | 1.140 | 0.864 | 0.988 |
| bL | 0.195 | 0.366 | 0.286 | |
| R2 | 0.987 | 0.993 | 0.983 | |
| AIC | −75 | −83 | −74 | |
| Freundlich | kF | 0.199 | 0.233 | 0.230 |
| nF | 1.54 | 1.77 | 1.70 | |
| R2 | 0.991 | 0.993 | 0.991 | |
| AIC | −82 | −85 | −81 | |
| Temkin | AT | 8.32 | 9.51 | 10.4 |
| bT | 18388 | 18348 | 18550 | |
| R2 | 0.893 | 0.943 | 0.908 | |
| AIC | −54 | −62 | −56 | |
| (b) | ||||
| Series # | ||||
| Model | Parameter | 1 | 2 | 3 |
| Experim. | qm,exp. | 1.86 | 1.84 | 1.91 |
| Langmuir | qeq,L | 2.02 | 2.02 | 2.13 |
| bL | 1.93 | 2.15 | 1.75 | |
| R2 | 0.993 | 0.995 | 0.994 | |
| AIC | −55 | −58 | −57 | |
| Freundlich | kF | 2.90 | 1.20 | 1.19 |
| nF | 1.67 | 2.92 | 2.77 | |
| R2 | 0.908 | 0.971 | 0.975 | |
| AIC | 28 | −42 | −43 | |
| Sips | qeq,S | 2.34 | 2.24 | 2.41 |
| bS | 1.21 | 1.47 | 1.21 | |
| nS | 1.34 | 1.26 | 1.27 | |
| R2 | 0.995 | 0.996 | 0.995 | |
| AIC | −58 | −61 | −58 | |
| Temkin | AT | 47.1 | 49.9 | 42.3 |
| bT | 7187 | 7114 | 6785 | |
| R2 | 0.985 | 0.988 | 0.982 | |
| AIC | −49 | −51 | −47 | |
| (c) | ||||
| Series # | ||||
| Model | Parameter | 1 | 2 | 3 |
| Experim. | qm,exp. | 2.40 | 2.28 | 2.34 |
| Langmuir | qeq,L | 2.38 | 2.27 | 2.33 |
| bL | 4.90 | 4.68 | 4.70 | |
| R2 | 0.968 | 0.969 | 0.988 | |
| AIC | −33 | −34 | −46 | |
| Freundlich | kF | 1.70 | 1.63 | 1.66 |
| nF | 3.66 | 3.64 | 3.55 | |
| R2 | 0.963 | 0.968 | 0.960 | |
| AIC | −33 | −36 | −33 | |
| Sips | qeq,S | 2.97 | 2.96 | 2.61 |
| bS | 1.69 | 1.48 | 2.51 | |
| nS | 1.69 | 1.80 | 1.37 | |
| R2 | 0.985 | 0.986 | 0.995 | |
| AIC | −40 | −42 | −52 | |
| Temkin | AT | 165.7 | 205.3 | 121.8 |
| bT | 7404 | 8081 | 7144 | |
| R2 | 0.987 | 0.985 | 0.995 | |
| AIC | −45 | −45 | −56 | |
| Sorbent | pH | Time (min) | qm,exp (mmol g−1) | qm,L (mmol g−1) | bL (L mmol−1) | Ref. |
|---|---|---|---|---|---|---|
| Dowex 50W8 sulfonic resin | 3.7 | 60 | 1.43 | 1.43 | 206 | [36] |
| Alginate microsphere | 6 | 1440 | 1.20 | 1.27 | 8.24 | [58] |
| Resorcinol-formaldehyde resin | 7 | 1440 | - | 1.14 | - | [34] |
| Sulfonated polyaniline sorbent | Nat. | 40 | 1.01 | 1.05 | 4.73 | [110] |
| Amidoximated algal/PEI beads | 6 | 90 | 2.16 | 2.36 | 2.01 | [38] |
| Crab carapace | Nat. | 240 | 0.038 | 0.045 | 11.4 | [111] |
| SrTreat® | Nat. | 60 | 0.104 | 0.109 | 265 | [111] |
| Kurion-TS™ | Nat. | 60 | 0.128 | 0.230 | 492 | [111] |
| Mixed-bed resin (T-46/A-33) | 7 | 30 | - | 0.109 | 0.084 | [35] |
| Functionalized silica beads | 8 | 60 | 1.38 | 1.57 | 1.41 | [74] |
| Magnetic composite sulfonated sorbent | 10 | 180 | 0.539 | - | - | [30] |
| Salvadora persica biomass | 7 | 60 | - | 0.474 | 0.237 | [27] |
| Fly ash-based zeolite | 5.4 | 720 | 0.681 | 0.749 | 0.756 | [112] |
| Photinia serrulata leaf | >4 | 30 | 0.120 | 0.138 | 11.0 | [113] |
| S. cerevisiae-Fe3O4 composite | 6 | 960 | - | 0.234 | 1.33 | [29] |
| Bacillus pumilus SWU7–1 | 7 | 7200 | 3.14 | 3.42 | 2.89 | [28] |
| Modified montmorillonite | 7 | 30 | - | 0.028 | 40.2 | [114] |
| Granular manganese oxide | - | 4800 | 1.2 | 1.9 | 0.1 | [73] |
| Zr-metal-organic framework | Nat. | 5 | - | 0.871 | 2.51 | [115] |
| Functionalized graphene oxide | 2 | 60 | 1.37 | 1.44 | 3.03 | [75] |
| SLS/polyacrylonitrile | 11.5 | 800 | 0.4 | 0.376 | 10.8 | [64] |
| PVA/graphene oxide aerogel | 7 | 480 | 0.228 | 0.229 | 239 | [116] |
| Graphene oxide | 5 | 20 | 0.97 | 1.50 | 0.850 | [76] |
| ZrSn(IV) phosphate nanocomp. | 8 | 120 | - | 0.202 | 3.94 | [117] |
| A*PEI | 5 | 120 | 0.607 | 0.977 | 0.278 | This study |
| SA*PEI | 5 | 40 | 1.91 | 2.05 | 1.94 | This study |


Appendix B.5. Sorption Selectivity




Appendix B.6. Sr(II) Desorption

Appendix B.7. Application to Seawater Samples
| Metal Ion | Units | Mediterranean Sea | Red Sea |
|---|---|---|---|
| Na(I) | g L−1 | 13.03 | 14.10 |
| K(I) | mg L−1 | 554.8 | 489.5 |
| Mg(II) | mg L−1 | 1424 | 1504 |
| Ca(II) | mg L−1 | 555.3 | 568.4 |
| Sr(II) | mg L−1 | 4.218 | 5.968 |
| B(III) | mg L−1 | 3.856 | 4.119 |
| U(VI) | µg L−1 | 9.8 | 10.9 |



Appendix C. Synthesis of Sorbents
Appendix C.1. Manufacturing of A*PEI Beads

Appendix C.2. Functionalization of A*PEI (Synthesis of SA*PEI)

References
- RSC. Periodic Table. Available online: https://www.rsc.org/periodic-table/ (accessed on 5 October 2021).
- Mangano, J.J.; Sternglass, E.J.; Gould, J.M.; Sherman, J.D.; Brown, J.; McDonnell, W. Strontium-90 in newborns and childhood disease. Arch. Environ. Health 2000, 55, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.M.; Sternglass, E.J.; Sherman, J.D.; Brown, J.; McDonnell, W.; Mangano, J.J. Strontium-90 in deciduous teeth as a factor in early childhood cancer. Int. J. Health Serv. 2000, 30, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Mangano, J.J.; Sherman, J.D. Elevated in vivo strontium-90 from nuclear weapons test fallout among cancer decedents: A case-control study of deciduous teeth. Int. J. Health Serv. 2011, 41, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Itoh, K.; Sugiyama, A.; Ichijo, S.; Ichijo, M.; Furuya, F.; Nakamura, Y.; Kitahara, K.; Kobayashi, K.; Yukawa, Y.; et al. Absorption of radionuclides from the Fukushima nuclear accident by a novel algal strain. PLoS ONE 2012, 7, e44200. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, N.O. The potential for disease initiation by inhaled beta-emitting nuclear particles. Med. Hypotheses 2018, 116, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Adigun, O.A.; Oninla, V.O.; Babarinde, N.A.; Oyedotun, K.O.; Manyala, N. Characterization of sugarcane leaf-biomass and investigation of its efficiency in removing nickel(II), chromium(III) and cobalt(II) ions from polluted water. Surf. Interfaces 2020, 20, 100621. [Google Scholar] [CrossRef]
- Shooto, N.D. Removal of toxic hexavalent chromium (Cr(VI)) and divalent lead (Pb(II)) ions from aqueous solution by modified rhizomes of Acorus Calamus. Surf. Interfaces 2020, 20, 100624. [Google Scholar] [CrossRef]
- Jayakumar, V.; Govindaradjane, S.; Rajasimman, M. Efficient adsorptive removal of zinc by green marine macro alga Caulerpa scalpelliformis—Characterization, optimization, modeling, isotherm, kinetic, thermodynamic, desorption and regeneration studies. Surf. Interfaces 2021, 22, 100798. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Dronov, M.; Koza, T.; Schwiers, A.; Schmidt, T.C.; Schram, J. Strontium carbonate precipitation as a sample preparation technique for isotope ratio analysis of Sr in mineral water and wine by quadrupole-based inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 149–158. [Google Scholar] [CrossRef]
- Thanh, L.H.V.; Liu, J.C. Flotation separation of strontium via phosphate precipitation. Water Sci. Technol. 2017, 75, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nhung, N.T.H.; Dai, X.; He, C.; Wang, Y.; Wei, Y.; Fujita, T. Strontium ion removal from artificial seawater using a combination of adsorption with biochar and precipitation by blowing CO2 nanobubble with neutralization. Front. Bioeng. Biotechnol. 2022, 10, 819407. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, T.; Towfighi, J.; Rezaei-Vahidian, H. Sorption of cesium and strontium ions by natural zeolite and management of produced secondary waste. Environ. Technol. Innov. 2020, 17, 100592. [Google Scholar] [CrossRef]
- Ogata, F.; Kobayashi, Y.; Uematsu, Y.; Nakamura, T.; Kawasaki, N. Zeolite produced from fly ash by thermal treatment in alkaline solution and Its capability to adsorb Cs(I) and Sr(II) in aqueous solution. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2020, 140, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Moamen, O.A.A.; Hassan, H.S.; Zaher, W.F. Taguchi L-16 optimization approach for simultaneous removal of Cs+ and Sr2+ ions by a novel scavenger. Ecotoxicol. Environ. Saf. 2020, 189, 110013. [Google Scholar] [CrossRef]
- Karmaker, S.C.; Eljamal, O.; Saha, B.B. Response surface methodology for strontium removal process optimization from contaminated water using zeolite nanocomposites. Environ. Sci. Pollut. Res. 2021, 28, 56535–56551. [Google Scholar] [CrossRef] [PubMed]
- Prajitno, M.Y.; Harbottle, D.; Hondow, N.; Zhang, H.; Hunter, T.N. The effect of pre-activation and milling on improving natural clinoptilolite for ion exchange of cesium and strontium. J. Environ. Chem. Eng. 2020, 8, 102991. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Ultrafast removal of radioactive strontium ions from contaminated water by nanostructured layered sodium vanadosilicate with high adsorption capacity and selectivity. J. Hazard. Mater. 2020, 398, 122907. [Google Scholar] [CrossRef]
- Jiao, Z.; Meng, Y.; He, C.; Yin, X.; Wang, X.; Wei, Y. One-pot synthesis of silicon-based zirconium phosphate for the enhanced adsorption of Sr (II) from the contaminated wastewater. Microporous Mesoporous Mater. 2021, 318, 111016. [Google Scholar] [CrossRef]
- Amesh, P.; Venkatesan, K.A.; Suneesh, A.S.; Maheswari, U. Tuning the ion exchange behavior of cesium and strontium on sodium iron titanate. Sep. Purif. Technol. 2021, 267, 118678. [Google Scholar] [CrossRef]
- Park, B.; Ghoreishian, S.M.; Kim, Y.; Park, B.J.; Kang, S.-M.; Huh, Y.S. Dual-functional micro-adsorbents: Application for simultaneous adsorption of cesium and strontium. Chemosphere 2021, 263, 128266. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; He, F.; Dai, Y.D. Prussian blue analog caged in chitosan surface-decorated carbon nanotubes for removal cesium and strontium. J. Radioanal. Nucl. Chem. 2016, 310, 1139–1145. [Google Scholar] [CrossRef]
- Ali, M.M.S.; Sami, N.M.; El-Sayed, A.A. Removal of Cs+, Sr2+ and Co2+ by activated charcoal modified with Prussian blue nanoparticle (PBNP) from aqueous media: Kinetics and equilibrium studies. J. Radioanal. Nucl. Chem. 2020, 324, 189–201. [Google Scholar] [CrossRef]
- El-Bahy, S.M.; Fadel, D.A.; El-Bahy, Z.M.; Metwally, A.M. Rapid and highly efficient cesium removal by newly synthesized carbomer encapsulated potassium copper hexacyanoferrate composite. J. Environ. Chem. Eng. 2018, 6, 1875–1885. [Google Scholar] [CrossRef]
- Vincent, C.; Barre, Y.; Vincent, T.; Taulemesse, J.M.; Robitzer, M.; Guibal, E. Chitin-Prussian blue sponges for Cs(I) recovery: From synthesis to application in the treatment of accidental dumping of metal-bearing solutions. J. Hazard. Mater. 2015, 287, 171–179. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Youssef, M.A.; Aboterika, A.H.A.; Awwad, N.S. Removal of barium and strontium from wastewater and radioactive wastes using a green bioadsorbent, Salvadora persica (Miswak). Desalin. Water Treat. 2020, 192, 306–314. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, T.; Zhao, Y.; Li, Q.; Dong, F.; Jiang, C. Potentiality of living Bacillus pumilus SWU7-1 in biosorption of strontium radionuclide. Chemosphere 2020, 260, 127559. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhao, X.; Zhou, H.; Qiu, L.; Dai, Y.; Luo, H.; Otero, M. Removal of strontium by high-performance adsorbents Saccharomyces cerevisiae-Fe3O4 bio-microcomposites. J. Radioanal. Nucl. Chem. 2020, 326, 525–535. [Google Scholar] [CrossRef]
- El-Saied, H.A.; El-Din, A.M.S.; Masry, B.A.; Ibrahim, A.M. A promising superabsorbent nanocomposite based on grafting biopolymer/nanomagnetite for capture of 134Cs, 85Sr and 60Co radionuclides. J. Polym. Environ. 2020, 28, 1749–1765. [Google Scholar] [CrossRef]
- Abou-Lilah, R.A.; Rizk, H.E.; Elshorbagy, M.A.; Gamal, A.M.; Ali, A.M.; Badawy, N.A. Efficiency of bentonite in removing cesium, strontium, cobalt and uranium ions from aqueous solution: Encapsulation with alginate for column application. Int. J. Environ. Anal. Chem. 2020, 102, 2913–2936. [Google Scholar] [CrossRef]
- Maqbool, M.; Sadaf, S.; Bhatti, H.N.; Rehmat, S.; Kausar, A.; Alissa, S.A.; Iqbal, M. Sodium alginate and polypyrrole composites with algal dead biomass for the adsorption of Congo red dye: Kinetics, thermodynamics and desorption studies. Surf. Interfaces 2021, 25, 101183. [Google Scholar] [CrossRef]
- Vorster, C.; van der Walt, T.N.; Coetzee, P.P. Ion exchange separation of strontium and rubidium on Dowex 50W-X8, using the complexation properties of EDTA and DCTA. Anal. Bioanal.Chem. 2008, 392, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nur, T.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Removal of strontium from aqueous solutions and synthetic seawater using resorcinol formaldehyde polycondensate resin. Desalination 2017, 420, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Malodia, P.; Kachwaha, M.; Verma, S. Adsorptive and kinetic studies of resin for removal of Cs+ and Sr2+ from aqueous solution. J. Water Chem. Technol. 2019, 41, 292–298. [Google Scholar] [CrossRef]
- Hafizi, M.; Abolghasemi, H.; Moradi, M.; Milani, S.A. Strontium adsorption from sulfuric acid solution by Dowex 50W-X resins. Chin. J. Chem. Eng. 2011, 19, 267–272. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Ignat, M.; Varganici, C.D. Superadsorbents for strontium and cesium removal enriched in amidoxime by a homo-IPN strategy connected with porous silica texture. ACS Appl. Mater. Interfaces 2020, 12, 44622–44638. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Salih, K.A.M.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime functionalization of algal/polyethyleneimine beads for the sorption of Sr(II) from aqueous solutions. Molecules 2019, 24, 3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nada, A.M.A.; El-Wakil, N.A.; Hassan, M.L.; Adel, A.M. Differential adsorption of heavy metal ions by cotton stalk cation-exchangers containing multiple functional groups. J. Appl. Polym. Sci. 2006, 101, 4124–4132. [Google Scholar] [CrossRef]
- Erenturk, S.A.; Haciyakupoglu, S.; Senkal, B.F. Investigation of interaction behaviours of cesium and strontium ions with engineering barrier material to prevent leakage to environmental. J. Environ. Radioact. 2020, 213, 106101. [Google Scholar] [CrossRef]
- Kwak, N.S.; Yang, J.R.; Hwang, C.W.; Hwang, T.S. The effect of a molecular weight and an amount of PEGDA (poly(ethylene glycol)diacrylate) on a preparation of sodium methallyl sulfonate-co-PEGDA microspheres and sorption behavior of Co(II). Chem. Eng. J. 2013, 223, 216–223. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Vasudevan, T.; Pandey, A.K.; Das, S.; Pujari, P.K. Poly(ethylene glycol methacrylate phosphate-co-2-acrylamido-2-methyl-1-propane sulfonate) pore-filled substrates for heavy metal ions sorption. Chem. Eng. J. 2014, 236, 9–16. [Google Scholar] [CrossRef]
- Yang, J.J.; Dong, Y.H.; Li, J.; Liu, Z.J.; Min, F.L.; Li, Y.Y. Removal of Co(II) from aqueous solutions by sulfonated magnetic multi-walled carbon nanotubes. Korean J. Chem. Eng. 2015, 32, 2247–2256. [Google Scholar] [CrossRef]
- Borai, E.H.; Hamed, M.G.; El-kamash, A.M.; Siyam, T.; El-Sayed, G.O. Synthesis, characterization and application of a modified acrylamide–styrene sulfonate resin and a composite for sorption of some rare earth elements. New J. Chem. 2015, 39, 7409–7420. [Google Scholar] [CrossRef]
- Parlak, E.; Arar, O. Removal of copper (Cu2+) from water by sulfonated cellulose. J. Disp. Sci. Technol. 2018, 39, 1403–1408. [Google Scholar] [CrossRef]
- Arar, O. Co-precipitative preparation of a sulfonated cellulose-magnetite hybrid sorbent for the removal of Cu2+ ions. Anal. Sci. 2020, 36, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.D.; Siriwardane, R.; McIntyre, D. Anion structural effects on interaction of rare earth element ions with Dowex 50W X8 cation exchange resin. J. Rare Earths 2018, 36, 879–890. [Google Scholar] [CrossRef]
- Hamza, M.F.; Lu, S.M.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; Wei, Y.Z.; Vincent, T.; Guibal, E. As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Sci. Total Environ. 2020, 719, 137396. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mubark, A.E.; Wei, Y.; Vincent, T.; Guibal, E. Quaternization of composite algal/PEI beads for enhanced uranium sorption-application to ore acidic leachate. Gels 2020, 6, 6020012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, M.F.; Wei, Y.; Guibal, E. Quaternization of algal/PEI beads (a new sorbent): Characterization and application to scandium recovery from aqueous solutions. Chem. Eng. J. 2020, 383, 123210. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Hamza, M.F.; Fujita, T.; Rodríguez-Castellón, E.; Guibal, E. Synthesis of a new phosphonate-based sorbent and characterization of its interactions with lanthanum (III) and terbium (III). Polymers 2021, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Salih, K.A.M.; Rabie, K.; Elwakeel, K.Z.; Zayed, Y.E.; Hamza, M.F.; Guibal, E. Development of phosphoryl-functionalized algal-PEI beads for the sorption of Nd(III) and Mo(VI) from aqueous solutions—Application for rare earth recovery from acid leachates. Chem. Eng. J. 2021, 412, 127399. [Google Scholar] [CrossRef]
- Zhang, Y.; Hamza, M.F.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Tuning the sorption properties of amidoxime-functionalized algal/polyethyleneimine beads for La(III) and Dy(III) using EDTA: Impact of metal speciation on selective separation. Chem. Eng. J. 2021, 431, 133214. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.M.; Abdel-Rahman, A.A.H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Demadis, K.D.; Paspalaki, M.; Theodorou, J. Controlled release of bis(phosphonate) pharmaceuticals from cationic biodegradable polymeric matrices. Ind. Eng. Chem. Res. 2011, 50, 5873–5876. [Google Scholar] [CrossRef]
- Haug, A. Dissociation of alginic acid. Acta Chem. Scand. 1961, 15, 950–952. [Google Scholar] [CrossRef]
- Hong, H.J.; Ryu, J.; Park, I.S.; Ryu, T.; Chung, K.S.; Kim, B.G. Investigation of the strontium (Sr(II)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater. J. Environ. Manag. 2016, 165, 263–270. [Google Scholar] [CrossRef]
- Dong, H.T.; Du, H.B.; Wickramasinghe, S.R.; Qian, X.H. The effects of chemical substitution and polymerization on the pKa values of sulfonic acids. J. Phys. Chem. B 2009, 113, 14094–14101. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mahfouz, M.G.; Abdel-Rahman, A.A.H. Adsorption of uranium (VI) ions on hydrazinyl amine and 1,3,4-thiadiazol-2(3 H)-thion chelating resins. J. Disp. Sci. Technol. 2012, 33, 1544–1551. [Google Scholar] [CrossRef]
- Lu, S.J.; Duan, M.L.; Lin, S.B. Synthesis of superabsorbent starch graft-poly(potassium acrylate-co-acrylamide) and its properties. J. Appl. Polym. Sci. 2003, 88, 1536–1542. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of kappa-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Hassan, R.S.; Rashad, G.M. One-pot synthesis of sodium lauryl sulfate-loaded polyacrylonitrile solid-phase extractor for investigating the adsorption behavior of radioactive strontium(II) from aqueous solutions. Appl. Radiat. Isot. 2020, 163, 109198. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.-G.; Kwak, J.; Kim, S.; Lee, S.-H.; Park, Y.; Lee, S.-D.; Chon, K. Adsorption of radioactive strontium by pristine and magnetic biochars derived from spent coffee grounds. J. Environ. Chem. Eng. 2021, 9, 105119. [Google Scholar] [CrossRef]
- Bezhin, N.A.; Dovhyi, I.I.; Tokar, E.A.; Tananaev, I.G. Physical and chemical regularities of cesium and strontium recovery from the seawater by sorbents of various types. J. Radioanal. Nucl. Chem. 2021, 330, 1101–1111. [Google Scholar] [CrossRef]
- Morig, C.R.; Gopala Rao, M. Diffusion in ion exchange resins: Sodium ion-strontium ion system. Chem. Eng. Sci. 1965, 20, 889–893. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakamoto, Y.; Senoo, M. Consideration on effective eiffusivity of strontium in granite. J. Nucl. Sci. Technol. 1993, 30, 796–803. [Google Scholar] [CrossRef]
- Severin, A.V.; Gopin, A.V.; Vasiliev, A.N.; Enikeev, K.I. Diffusion and sorption of radium and strontium in a layer of porous sorbent based on hydroxyapatite. Radiochemistry 2021, 63, 51–55. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.J.; Li, J.S.; Sun, X.Y.; Zhang, L. Synthesis of modified D401 chelating resin and its adsorption properties for Pb2+. J. Cent. South Univ. Technol. 2009, 16, 575–580. [Google Scholar] [CrossRef]
- Chu, K.H. Revisiting the Temkin isotherm: Dimensional inconsistency and approximate forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Maruyama, H.; Seki, H. Adsorption modeling by two sites Langmuir type isotherm for adsorption of bisphenol-A and diethyl phthalate onto activated carbon in single and binary system. Sep. Sci. Technol. 2022, 57, 1535–1542. [Google Scholar] [CrossRef]
- Matskevich, A.I.; Tokar, E.A.; Ivanov, N.P.; Sokolnitskaya, T.A.; Parot’kina, Y.A.; Dran’kov, A.N.; Silant’ev, V.E.; Egorin, A.M. Study on the adsorption of strontium on granular manganese oxide. J. Radioanal. Nucl. Chem. 2021, 327, 1005–1017. [Google Scholar] [CrossRef]
- Wei, Y.; Rakhatkyzy, M.; Salih, K.A.M.; Wang, K.; Hamza, M.F.; Guibal, E. Controlled bi-functionalization of silica microbeads through grafting of amidoxime/methacrylic acid for Sr(II) enhanced sorption. Chem. Eng. J. 2020, 402, 125220. [Google Scholar] [CrossRef]
- Alamdarlo, F.V.; Solookinejad, G.; Zahakifar, F.; Jalal, M.R.; Jabbari, M. Study of kinetic, thermodynamic, and isotherm of Sr adsorption from aqueous solutions on graphene oxide (GO) and (aminomethyl)phosphonic acid-graphene oxide (AMPA-GO). J. Radioanal. Nucl. Chem. 2021, 329, 1033–1043. [Google Scholar] [CrossRef]
- Abu-Nada, A.; Abdala, A.; McKay, G. Isotherm and kinetic modeling of strontium adsorption on graphene oxide. Nanomaterials 2021, 11, 2780. [Google Scholar] [CrossRef] [PubMed]
- Bonner, O.D.; Smith, L.L. A selectivity scale for some divalent cations on Dowex 50. J. Phys. Chem. 1957, 61, 326–329. [Google Scholar] [CrossRef]
- Iyer, S.T.; Nandan, D.; Venkataramani, B. Alkaline earth metal ion-proton-exchange equilibria on Nafion-117 and Dowex 50W X8 in aqueous solutions at 298+/−1K. React. Funct. Polym. 1996, 29, 51–57. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+, and Pb2+ from aqueous solution on Dowex 50W synthetic resin. J. Hazard. Mater. 2006, 134, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.R. Molecular level interpretation of ion-exchange selectivity. Indian J. Chem. Sect A 1990, 29, 409–417. [Google Scholar]
- Yin, J.; Yang, S.; He, W.; Zhao, T.; Li, C.; Hua, D. Biogene-derived aerogels for simultaneously selective adsorption of uranium(VI) and strontium(II) by co-imprinting method. Sep. Purif. Technol. 2021, 271, 118849. [Google Scholar] [CrossRef]
- Kirishima, A.; Sasaki, T.; Sato, N. Solution chemistry study of radioactive Sr on Fukushima Daiichi NPS site. J. Nucl. Sci. Technol. 2015, 52, 152–161. [Google Scholar] [CrossRef]
- Amphlett, J.T.M.; Choi, S.; Parry, S.A.; Moon, E.M.; Sharrad, C.A.; Ogden, M.D. Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: Chelation vs. anion exchange. Chem. Eng. J. 2020, 392, 123712. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Virolainen, E.; Conley, K.; Vahala, R. Chitosan-zinc(II) complexes as a bio-sorbent for the adsorptive abatement of phosphate: Mechanism of complexation and assessment of adsorption performance. Polymers 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Maurin, G.; Llewellyn, P. (Eds.) 1—Introduction. In Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press: Oxford, UK, 2014; pp. 1–24. [Google Scholar]
- Sing, K.S.W.; Rouquerol, F.; Rouquerol, J.; Llewellyn, P. 8—Assessment of mesoporosity. In Adsorption by Powders and Porous Solids, 2nd ed.; Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G., Eds.; Academic Press: Oxford, UK, 2014; pp. 269–302. [Google Scholar]
- Sato, D.M.; Guerrini, L.M.; de Oliveira, M.P.; de Oliveira Hein, L.R.; Botelho, E.C. Production and characterization of polyetherimide mats by an electrospinning process. Mater. Res. Express 2018, 5, 115302. [Google Scholar] [CrossRef] [Green Version]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef]
- Sui, C.; Preece, J.A.; Zhang, Z. Novel polystyrene sulfonate-silica microspheres as a carrier of a water soluble inorganic salt (KCl) for its sustained release, via a dual-release mechanism. RSC Adv. 2017, 7, 478–481. [Google Scholar] [CrossRef] [Green Version]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Pinilla-Torres, A.M.; Carrion-Garcia, P.Y.; Sanchez-Dominguez, C.N.; Gallardo-Blanco, H.; Sanchez-Dominguez, M. Modification of branched polyethyleneimine using Mesquite Gum for its improved hemocompatibility. Polymers 2021, 13, 2766. [Google Scholar] [CrossRef]
- Zaaeri, F.; Khoobi, M.; Rouini, M.; Javar, H.A. pH-responsive polymer in a core-shell magnetic structure as an efficient carrier for delivery of doxorubicin to tumor cells. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 967–977. [Google Scholar] [CrossRef]
- Zhengbo, H.; Zhu, W.; Song, H.; Chen, P.; Yao, S. The adsorption behavior and mechanism investigation of Cr(VI) ions removal by poly(2-(dimethylamino)ethyl methacrylate)/poly(ethyleneimine) gels. J. Serb. Chem. Soc. 2015, 80, 889–902. [Google Scholar]
- Xu, Z.P.; Braterman, P.S. High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J. Mater. Chem. 2003, 13, 268–273. [Google Scholar] [CrossRef]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grondahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Dangi, Y.R.; Bediako, J.K.; Lin, X.; Choi, J.-W.; Lim, C.-R.; Song, M.-H.; Han, M.; Yun, Y.-S. Polyethyleneimine impregnated alginate capsule as a high capacity sorbent for the recovery of monovalent and trivalent gold. Sci. Rep. 2021, 11, 17836. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Ojha, K.; Dey, S.; Mandal, A. Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from Sapindus laurifolius. RSC Adv. 2018, 8, 24485–24499. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.R.; Daskalakis, N.; Demadis, K.D.; Mezei, G. Cation effect on the inorganic-organic layered structure of pyrazole-4-sulfonate networks and inhibitory effects on copper corrosion. New J. Chem. 2010, 34, 221–235. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Uber die adsorption in lasungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Newton, MA, USA, 1994; p. 243. [Google Scholar]
- Kegl, T.; Kosak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]
- Puigdomenech, I. MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms); 32 Bit Version; Royal Institute of Technology: Stockholm, Sweden, 2010. [Google Scholar]
- AFSSA. Guidelines on the Assessment of Ion Exchangers Used for the Treatment of Water Intended for Human Consumption; AFSSA: Maisons-Alfort, France, 2009; p. 60. [Google Scholar]
- Lu, T.T.; Zhu, Y.F.; Wang, W.B.; Qi, Y.X.; Wang, A.Q. Polyaniline-functionalized porous adsorbent for Sr2+ adsorption. J. Radioanal. Nucl. Chem. 2018, 317, 907–917. [Google Scholar] [CrossRef]
- Rae, I.B.; Pap, S.; Svobodova, D.; Gibb, S.W. Comparison of sustainable biosorbents and ion-exchange resins to remove Sr2+ from simulant nuclear wastewater: Batch, dynamic and mechanism studies. Sci. Total Environ. 2019, 650, 2411–2422. [Google Scholar] [CrossRef]
- Lihareva, N.; Petrov, O.; Dimowa, L.; Tzvetanova, Y.; Piroeva, I.; Ublekov, F.; Nikolov, A. Ion exchange of Cs+ and Sr2+ by natural clinoptilolite from bi-cationic solutions and XRD control of their structural positioning. J. Radioanal. Nucl. Chem. 2020, 323, 1093–1102. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Bai, X.; Lv, W.; Liao, Y. Adsorption of strontium onto adaxial and abaxial cuticle of Photinia serrulata leaf. Int. J. Environ. Res. Public Health 2020, 17, 1061. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Verma, S.; Harwani, G.; Patidar, D. Adsorptive and kinetic studies of the extraction of toxic metal ion from contaminated water using modified montmorillonites. J. Water Chem. Technol. 2021, 43, 321–329. [Google Scholar] [CrossRef]
- Ren, L.; Zhao, X.; Liu, B.; Huang, H. Synergistic effect of carboxyl and sulfate groups for effective removal of radioactive strontium ion in a Zr-metal-organic framework. Water Sci. Technol. 2021, 83, 2001–2011. [Google Scholar] [CrossRef]
- Huo, J.-B.; Yu, G.; Wang, J. Adsorptive removal of Sr(II) from aqueous solution by polyvinyl alcohol/graphene oxide aerogel. Chemosphere 2021, 278, 130492. [Google Scholar] [CrossRef]
- Abass, M.R.; Maree, R.M.; Sami, N.M. Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. Int. J. Environ. Anal. Chem. 2022, 2016728. [Google Scholar] [CrossRef]
- Mahmoud, S.A.E.A.; Mosleh, N.M.; Mansour, G.M.R. The Zug El Bohar hydrothermal uranium-base metal deposits, Southwest El Quseir, Red Sea Coast, Egypt. Arab J. Nucl. Sci. Appl. 2018, 51, 228–247. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, S.A.E.A.; Gehad, M.R.M. The hydrothermal uranium and some other metal deposits of the extensional faulting during the advanced opening of the Red Sea, Central Eastern Desert, Egypt. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 752–766. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.M.; Zhou, K.; Wei, Y.; Abu Khoziem, H.A.; Alotaibi, S.H.; Guibal, E. Effect of bi-functionalization of algal/polyethyleneimine composite beads on the enhancement of tungstate sorption: Application to metal recovery from ore leachate. Sep. Purif. Technol. 2022, 290, 120893. [Google Scholar] [CrossRef]
- Wallace, R.G. Hydroxylamine-O-sulfonic acid—A versatile synthetic reagent. Aldrichimica Acta 1980, 13, 3–11. [Google Scholar]










| Sorbent | A*PEI | SA*PEI | |||
|---|---|---|---|---|---|
| Model | Parameter | Temperature | T: 20 ± 1 °C | T: 20 ± 1 °C | T: 50 ± 1 °C |
| Unit | |||||
| Experimental | qeq | mmol Sr g−1 | 0.179 | 0.926 | 1.17 |
| PFORE | qeq,1 | mmol Sr g−1 | 0.198 | 0.974 | 1.23 |
| k1 × 102 | min−1 | 2.04 | 5.01 | 5.80 | |
| R2 | - | 0.969 | 0.976 | 0.977 | |
| AIC | - | −144 | −96 | −91 | |
| PSORE | qeq,2 | mmol Sr g−1 | 0.268 | 1.15 | 1.43 |
| k2 × 102 | g mmol−1 min−1 | 26.8 | 4.93 | 4.85 | |
| R2 | - | 0.952 | 0.938 | 0.937 | |
| AIC | - | −139 | −83 | −77 | |
| RIDE | De × 109 | m2 min−1 | 6.86 | 7.13 | 6.36 |
| R2 | - | 0.944 | 0.940 | 0.936 | |
| AIC | - | −133 | −82 | −75 | |
| Sorbent | A*PEI | SA*PEI | ||
|---|---|---|---|---|
| Temperature | 20 ± 1 °C | 20 ± 1 °C | 50 ± 1 °C | |
| Model | Parameter | |||
| Experim. | qm,exp. | 0.607 | 1.91 | 2.40 |
| Langmuir | qeq,L | 0.977 | 2.05 | 2.33 |
| bL | 0.278 | 1.94 | 4.88 | |
| R2 | 0.981 | 0.992 | 0.969 | |
| AIC | −214 | −164 | −110 | |
| Langmuir dual site | qeq,L1 | - | 0.192 | 0.903 |
| bL1 | - | 63.4 | 34.0 | |
| qeq,L2 | - | 1.94 | 1.65 | |
| bL2 | - | 1.41 | 1.36 | |
| R2 | - | 0.994 | 0.986 | |
| AIC | - | −180 | −136 | |
| Freundlich | kF | 0.221 | 1.18 | 1.66 |
| nF | 1.67 | 2.87 | 3.61 | |
| R2 | 0.986 | 0.968 | 0.956 | |
| AIC | −231 | −128 | −104 | |
| Sips | qeq,S | - | 2.33 | 2.83 |
| bS | - | 1.29 | 1.83 | |
| nS | - | 1.29 | 1.62 | |
| R2 | - | 0.994 | 0.984 | |
| AIC | - | −177 | −134 | |
| Temkin | AT | 9.37 | 46.4 | 162 |
| bT | 18,440 | 7028 | 7552 | |
| R2 | 0.899 | 0.981 | 0.984 | |
| AIC | −168 | −145 | −118 | |
| Temkin-II | qT | - | 0.434 | 0.395 |
| KT | - | 18.2 | 92.2 | |
| R2 | - | 0.991 | 0.984 | |
| AIC | - | −168 | −137 | |
| Sorbent | A*PEI | SA*PEI | ||||||
|---|---|---|---|---|---|---|---|---|
| Cycle | SE (%) | DE (%) | SE (%) | DE (%) | ||||
| Aver. | St. dev. | Aver. | St. dev. | Aver. | St. dev. | Aver. | St. dev. | |
| #1 | 10.73 | 0.85 | 100.5 | 0.4 | 58.7 | 1.8 | 99.9 | 0.2 |
| #2 | 10.22 | 0.85 | 104.9 | 7.6 | 58.6 | 1.8 | 100.0 | 0.1 |
| #3 | 9.86 | 0.61 | 100.1 | 0.6 | 58.3 | 1.7 | 100.0 | 0.1 |
| #4 | 9.75 | 0.65 | 99.7 | 0.1 | 57.8 | 1.8 | 100.0 | 0.0 |
| #5 | 9.46 | 1.01 | 100.2 | 0.3 | 57.7 | 1.8 | 100.0 | 0.0 |
| Loss (5th/1st) | 11.8% | S and C | 1.7% | S and C | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.F.; Guibal, E.; Althumayri, K.; Vincent, T.; Yin, X.; Wei, Y.; Li, W. New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater. Molecules 2022, 27, 7128. https://doi.org/10.3390/molecules27207128
Hamza MF, Guibal E, Althumayri K, Vincent T, Yin X, Wei Y, Li W. New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater. Molecules. 2022; 27(20):7128. https://doi.org/10.3390/molecules27207128
Chicago/Turabian StyleHamza, Mohammed F., Eric Guibal, Khalid Althumayri, Thierry Vincent, Xiangbiao Yin, Yuezhou Wei, and Wenlong Li. 2022. "New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater" Molecules 27, no. 20: 7128. https://doi.org/10.3390/molecules27207128
APA StyleHamza, M. F., Guibal, E., Althumayri, K., Vincent, T., Yin, X., Wei, Y., & Li, W. (2022). New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater. Molecules, 27(20), 7128. https://doi.org/10.3390/molecules27207128