Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices
Abstract
:1. Introduction
2. Results and Discussion
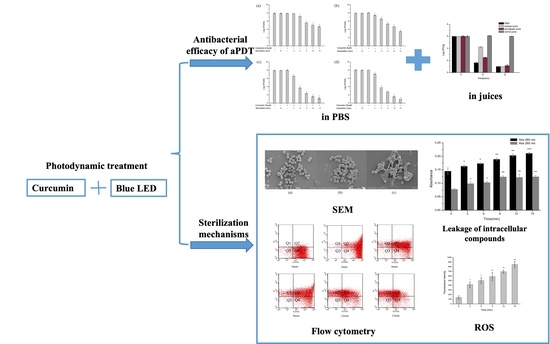
2.1. Antimicrobial Efficacy of aPDT in PBS against S. aureus
2.2. Inactivation Kinetics of aPDT in PBS against S. aureus
2.3. Change of Cell Morphology of S. aureus after aPDT
2.4. Effect of aPDT on Membrane Permeability of S. aureus
2.5. Effect of aPDT on Membrane Integrity and Esterase Activity of S. aureus
2.6. Effect of aPDT on Reactive Oxygen Species (ROS) of S. aureus
2.7. Antibacterial Efficacy of aPDT in Juices against S. aureus
2.8. Effects of the aPDT on the Color Changes of Juice
3. Materials and Methods
3.1. Bacterial Strains and Culture Conditions
3.2. Photosensitizer and Light Sources
3.3. Antibacterial Efficacy of aPDT in PBS
3.4. Inactivation Kinetics of aPDT
3.5. Morphology Alteration of S. aureus Observed by Scanning Electron Microscopy (SEM)
3.6. Measurement of Intracellular Protein and DNA Leakage
3.7. Flow Cytometry for Observation of S. aureus Membrane Integrity and Esterase Activity
3.8. Change of Reactive Oxygen Species (ROS) of S. aureus after aPDT
3.9. Antibacterial Efficacy of aPDT in Juices
3.10. Color Analysis of Juice after aPDT
3.11. Statistic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, J.J.; Qu, L.B.; Bi, Y.F.; Pan, C.X.; Yang, R.; Zeng, H.J. Antibacterial activity and mechanism of chloroform fraction from aqueous extract of mugwort leaves (Artemisia argyi L.) against Staphylococcus aureus. Lett. Appl. Microbiol. 2022, 74, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Tahi, A.A.; Sousa, S.; Madani, K.; Silva, C.L.M.; Miller, F.A. Ultrasound and heat treatment effects on Staphylococcus aureus cell viability in orange juice. Ultrason. Sonochemistry 2021, 78, 105743. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naitali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Eversley, T.; Fillion, K.; MacLaurin, T.; Powell, D. Assessment of Food Safety Practices of Food Service Food Handlers (Risk Assessment Data): Testing a Communication Intervention (Evaluation of Tools). J. Food Prot. 2010, 73, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Motarjemi, Y.; Kaferstein, F.; Moy, G.; Quevedo, F. Contaminated weaning food: A major risk factor for diarrhoea and asso-ciated malnutrition. Bull. World Health Organ. 1993, 71, 79–92. [Google Scholar]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-contamination and recontamination by Salmonella in foods: A review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Wei, G.; Yang, G.; Wang, Y.; Jiang, H.; Fu, Y.; Yue, G.; Ju, R. Phototherapy-based combination strategies for bacterial infection treatment. Theranostics 2020, 10, 12241–12262. [Google Scholar] [CrossRef]
- Aurum, F.S.; Nguyen, L.T. Efficacy of photoactivated curcumin to decontaminate food surfaces under blue light emitting diode. J. Food Process Eng. 2019, 42, e12988. [Google Scholar] [CrossRef]
- Chen, B.W.; Huang, J.M.; Liu, Y.; Liu, H.Q.; Zhao, Y.; Wang, J.J. Effects of the curcumin-mediated photodynamic inactivation on the quality of cooked oysters with Vibrio parahaemolyticus during storage at different temperature. Int. J. Food Microbiol. 2021, 345, 109152. [Google Scholar] [CrossRef]
- Chen, B.; Huang, J.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Eradication of planktonic Vibrio parahaemolyticus and its sessile biofilm by curcumin-mediated photodynamic inactivation. Food Control 2020, 113, 107181. [Google Scholar] [CrossRef]
- Hyun, J.E.; Lee, S.Y. Antibacterial effect and mechanisms of action of 460-470nm light-emitting diode against Listeria mono-cytogenes and Pseudomonas fluorescens on the surface of packaged sliced cheese. Food Microbiol. 2020, 86, 103314. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. The effect of photosensitization mediated by curcumin on storage life of fresh date (Phoenix dactylifera L.) fruit. Food Control 2018, 93, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Ghate, V.S.; Zhou, W.; Yuk, H.G. Perspectives and Trends in the Application of Photodynamic Inactivation for Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Song, Y.; Pei, J.; Xue, F.; Cui, X.; Xiong, X.; Li, C. The application of photodynamic inactivation to microorganisms in food. Food Chem. X 2021, 12, 100150. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, e1801350. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Rocha, S.; Cunha, Â.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Effect of Photodynamic Therapy on the Virulence Factors of Staphylococcus aureus. Front. Microbiol. 2016, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, P.; Qiao, Y.; Kang, Y.; Yan, C.; Yu, Z.; Wang, J.; He, X.; Wu, H. pH responsive superporogen combined with PDT based on poly Ce6 ionic liquid grafted on SiO2 for combating MRSA biofilm infection. Theranostics 2020, 10, 4795–4808. [Google Scholar] [CrossRef]
- Lai, D.N.; Zhou, A.R.; Tan, B.K.; Tang, Y.B.; Hamzah, S.S.; Zhang, Z.G.; Lin, S.L.; Hu, J.M. Preparation and photodynamic bactericidal effects of curcumin-ll-cyclodextrin complex. Food Chem. 2021, 361, 130117. [Google Scholar] [CrossRef]
- Kim, M.J.; Yuk, H.G. Antibacterial Mechanism of 405-Nanometer Light-Emitting Diode against Salmonella at Refrigeration Temperature. Appl. Environ. Microbiol. 2017, 83, e02582-16. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Zhang, F.; Liu, B.; Chen, X.; Meng, X. Antibacterial mechanism and preservation effect of curcumin-based photody-namic extends the shelf life of fresh-cut pears. LWT Food Sci. Technol. 2021, 142, 110941. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Zhang, N.; Zhou, Q.; Fan, D.; Wang, M. Lipophilized apigenin derivatives produced during the frying process as novel antioxidants. Food Chem. 2022, 379, 132178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, L.; Zhu, Y.; Liu, N.; Fan, D.; Wang, M.; Zhao, Y. Quercetin Inhibited the Formation of Lipid Oxidation Products in Thermally Treated Soybean Oil by Trapping Intermediates. J. Agric. Food Chem. 2021, 69, 3479–3488. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegge, A.B.; Nielsen, T.T.; Larsen, K.L.; Bruzell, E.; Tonnesen, H.H. Impact of curcumin supersaturation in antibacterial photodynamic therapyueffect of cyclodextrin type and amount: Studies on curcumin and curcuminoides XLV. J. Pharm. Sci. 2012, 101, 1524–1537. [Google Scholar] [CrossRef]
- Jackson, Z.; Meghji, S.; MacRobert, A.; Henderson, B.; Wilson, M. Killing of the yeast and hyphal forms of Candida albicans using a light-activated antimicrobial agent. Lasers Med. Sci. 1999, 14, 150–157. [Google Scholar] [CrossRef]
- Le, T.D.; Phasupan, P.; Nguyen, L.T. Antimicrobial photodynamic efficacy of selected natural photosensitizers against food pathogens: Impacts and interrelationship of process parameters. Photodiagnosis Photodyn. Ther. 2020, 32, 102024. [Google Scholar] [CrossRef]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules 2022, 27, 5084. [Google Scholar] [CrossRef]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef]
- Hossain, M.S.; Rahman, N.N.N.A.; Balakrishnan, V.; Rajion, Z.A.; Ab. Kadir, M.O. Mathematical modeling of Enterococcus faecalis, Escherichia coli, and Bacillus sphaericus inactivation in infectious clinical solid waste by using steam autoclaving and supercritical fluid carbon dioxide sterilization. Chem. Eng. J. 2015, 267, 221–234. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, R.; Liu, Q.; Yan, H.; Zhang, Y.; Wang, S.; Yuan, Y. Antibacterial activity and mechanism of high voltage electro-static field (HVEF) against Staphylococcus aureus in medium plates and food systems. Food Control 2021, 120, 107566. [Google Scholar] [CrossRef]
- Buchovec, I.; Lukseviciute, V.; Kokstaite, R.; Labeikyte, D.; Kaziukonyte, L.; Luksiene, Z. Inactivation of Gram (-) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactiva-tion efficiency. J. Photochem. Photobiol. B Biol. 2017, 172, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2000, 3, 3–8. [Google Scholar]
- Lin, S.L.; Hu, J.M.; Tang, S.S.; Wu, X.Y.; Chen, Z.Q.; Tang, S.Z. Photodynamic Inactivation of Methylene Blue and Tungsten-Halogen Lamp Light against Food Pathogen Listeria monocytogenes. Photochem. Photobiol. 2012, 88, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Lin, S.L.; Tan, B.K.; Hamzah, S.S.; Lin, Y.; Kong, Z.H.; Zhang, Y.; Zheng, B.D.; Zeng, S.X. Photodynamic inactivation of Burkholderia cepacia by curcumin in combination with EDTA. Food Res. Int. 2018, 111, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, A.W.; Wang, X.N.; Zhang, H.W.; Xu, C.S. Inactivation of Staphylococcus aureus by photodynamic action of hypocrellin B. Photodiagnosis Photodyn. Ther. 2013, 10, 600–606. [Google Scholar] [CrossRef]
- Li, J.; Suo, Y.; Liao, X.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017, 39, 101–110. [Google Scholar] [CrossRef]
- Thomas, M.; Craik, J.D.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Amphiphilic cationic Zn-porphyrins with high pho-todynamic antimicrobial activity. Future Microbiol. 2015, 10, 709–724. [Google Scholar] [CrossRef]
- Baltazar, L.D.; Soares, B.M.; Carneiro, H.C.S.; Avila, T.V.; Gouveia, L.F.; Souza, D.G.; Ferreira, M.V.L.; Pinotti, M.; Santos, D.D.; Cisalpino, P.S. Photodynamic inhibition of Trichophyton rubrum: In vitro activity and the role of oxidative and nitrosative bursts in fungal death. J. Antimicrob. Chemother. 2013, 68, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Antimicrobial mechanism of pulsed light for the control of Escherichia coli O157:H7 and its application in carrot juice. Food Control 2019, 106, 106751. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Ávila-Sosa, R.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ruiz-López, I.I.; Guerrero-Beltrán, J.Á. Mathematical Modeling Used to Evaluate the Effect of UV-C Light Treatment on Microorganisms in Liquid Foods. Food Eng. Rev. 2020, 12, 290–308. [Google Scholar] [CrossRef]
- Gomez, P.L.; Welti-Chanes, J.; Alzamora, S.M. Hurdle technology in fruit processing. Annu. Rev. Food Sci. Technol. 2011, 2, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef] [PubMed]
- Gayan, E.; Serrano, M.J.; Alvarez, I.; Condon, S. Modeling optimal process conditions for UV-heat inactivation of foodborne pathogens in liquid foods. Food Microbiol. 2016, 60, 13–20. [Google Scholar] [CrossRef]
- Baysal, A.H.; Molva, C.; Unluturk, S. UV-C light inactivation and modeling kinetics of Alicyclobacillus acidoterrestris spores in white grape and apple juices. Int. J. Food Microbiol. 2013, 166, 494–498. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, A.; Kuraya, E.; Touyama, A.; Higa, O.; Hokamoto, K.; Itoh, S. Underwater shockwave pretreatment process for improving carotenoid content and yield of extracted carrot (Daucus carota L.) juice. J. Food Eng. 2017, 211, 15–21. [Google Scholar] [CrossRef]
- Lin, Y.L.; Hu, J.M.; Li, S.Y.; Hamzah, S.S.; Jiang, H.Q.; Zhou, A.R.; Zeng, S.X.; Lin, S.L. Curcumin-Based Photodynamic Sterilization for Preservation of Fresh-Cut Hami Melon. Molecules 2019, 24, 2374. [Google Scholar] [CrossRef] [Green Version]
- Sheng, L.; Zhang, Z.; Sun, G.; Wang, L. Light-driven antimicrobial activities of vitamin K3 against Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Enteritidis. Food Control 2020, 114, 107235. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’ Donnell, C.P.; Muthukumarappan, K.; Cullen, P.J. Effect of sonication on orange juice quality parameters during storage. Int. J. Food Sci. Technol. 2009, 44, 586–595. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.; Min-Tze, L.; Karim, A.A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Bhavya, M.L.; Shewale, S.R.; Rajoriya, D.; Hebbar, H.U. Impact of Blue LED Illumination and Natural Photosensitizer on Bacterial Pathogens, Enzyme Activity and Quality Attributes of Fresh-Cut Pineapple Slices. Food Bioprocess Technol. 2021, 14, 362–372. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Zhou, W.; Yuk, H.G. Irradiance and Temperature Influence the Bactericidal Effect of 460-Nanometer Light-Emitting Diodes on Salmonella in Orange Juice. J. Food Prot. 2016, 79, 553–560. [Google Scholar] [CrossRef]
- Kaavya, R.; Pandiselvam, R.; Abdullah, S.; Sruthi, N.U.; Jayanath, Y.; Ashokkumar, C.; Chandra Khanashyam, A.; Kothakota, A.; Ramesh, S.V. Emerging non-thermal technologies for decontamination of Salmonella in food. Trends Food Sci. Technol. 2021, 112, 400–418. [Google Scholar] [CrossRef]
- Prasad, A.; Ganzle, M.; Roopesh, M.S. Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes. Foods 2019, 8, 679. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.; Liu, C.C.; Deng, H.; Li, J.; Ma, W.P.; Zeng, X.Y.; Ji, Y.H. In vitro photodynamic inactivation effects of hypocrellin B on azole-sensitive and resistant Candida albicans. Photodiagnosis Photodyn. Ther. 2019, 27, 419–427. [Google Scholar] [CrossRef]
- Huang, M.M.; Zhuang, H.; Zhao, J.Y.; Wang, J.M.; Yan, W.J.; Zhang, J.H. Differences in cellular damage induced by dielec-tric barrier discharge plasma between Salmonella Typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Mandro, G.F.; Tremarin, A.; Brandao, T.R.S.; Silva, C.L.M. UV-C light processing of Cantaloupe melon juice: Evaluation of the impact on microbiological, and some quality characteristics, during refrigerated storage. LWT Food Sci. Technol. 2019, 103, 247–252. [Google Scholar] [CrossRef]







| Initial Density (CFU/mL) | A | Kdm (min−1) | λ (min) | tt (min) | R2 | RMSE |
|---|---|---|---|---|---|---|
| 1 × 108 | 16.15 | 2.422 | 1.217 | 7.885 | 0.9987 | 0.2467 |
| 1 × 107 | 13.74 | 2.471 | 0.927 | 6.487 | 0.9977 | 0.2529 |
| 1 × 106 | 11.53 | 2.821 | 1.158 | 5.245 | 0.9998 | 0.063 |
| 1 × 105 | 9.308 | 2.948 | 1.212 | 4.369 | 0.999 | 0.1114 |
| 1 × 104 | 7.082 | 3.493 | 0.924 | 2.952 | 0.9999 | 0.0256 |
| Sample | Treatment | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| Mango juice | control | 58.9 ± 0.01 b | 3.59 ± 0.021 b | 42.17 ± 0.14 a | |
| 1.296 J/cm2 | 59.4 ± 0.059 a | 3.83 ± 0.075 a | 35.18 ± 0.046 b | 7.01 ± 0.112 | |
| 2.592 J/cm2 | 59.3 ± 0.023 a | 3.69 ± 0.012 a | 31.86 ± 0.058 b | 10.32 ± 0.085 | |
| Pineapple juice | control | 79.1 ± 0.064 b | −1.72 ± 0.025 b | 30.99 ± 0.235 a | |
| 1.296 J/cm2 | 80.1 ± 0.071 a | −0.60 ± 0.025 a | 22.40 ± 0.214 b | 8.72 ± 0.0601 | |
| 2.592 J/cm2 | 79.3 ± 0.127 a | −0.02 ± 0.046 a | 18.56 ± 0.125 b | 12.54 ± 0.139 | |
| Carrot juice | control | 16.1 ± 0.001 | 22.7 ± 0.001 | 20.3 ± 0.001 | |
| 1.296 J/cm2 | 16.1 ± 0.006 | 22.7 ± 0.025 | 20.6 ± 0.315 | 0.34 ± 0.316 | |
| 2.592 J/cm2 | 16.1 ± 0.001 | 22.7 ± 0.018 | 20.4 ± 0.116 | 0.07 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Liu, Q.; Huang, Y.; Qi, M.; Yan, H.; Li, W.; Zhuang, H. Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices. Molecules 2022, 27, 7136. https://doi.org/10.3390/molecules27207136
Yuan Y, Liu Q, Huang Y, Qi M, Yan H, Li W, Zhuang H. Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices. Molecules. 2022; 27(20):7136. https://doi.org/10.3390/molecules27207136
Chicago/Turabian StyleYuan, Yuan, Qingyan Liu, Yanjun Huang, Mengyuan Qi, Haiyang Yan, Wenliang Li, and Hong Zhuang. 2022. "Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices" Molecules 27, no. 20: 7136. https://doi.org/10.3390/molecules27207136
APA StyleYuan, Y., Liu, Q., Huang, Y., Qi, M., Yan, H., Li, W., & Zhuang, H. (2022). Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices. Molecules, 27(20), 7136. https://doi.org/10.3390/molecules27207136