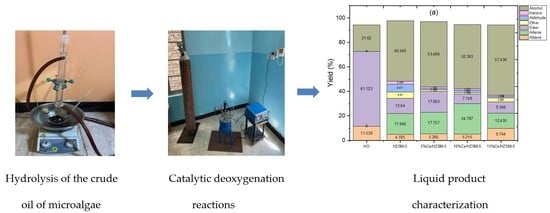
Ce-Loaded HZSM-5 Composite for Catalytic Deoxygenation of Algal Hydrolyzed Oil into Hydrocarbons and Oxygenated Compounds
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Extraction of the Crude Oil from Chlorella Vulgaris Microalgae
2.3. Hydrolysis of the Crude Oil of Chlorella Vulgaris Microalgae
2.4. Catalyst Preparation
2.5. Catalyst Characterization
2.6. Catalytic Deoxygenation of the Alga HO Using the Parent HZSM-5, and Lanthanum Modified Zeolites
2.7. Product Analysis
3. Results and Discussions
3.1. Catalyst Characterization
3.1.1. XRD Results
3.1.2. Surface Analysis
3.1.3. Ammonia TPD Analysis
3.1.4. Thermogravimetric Analysis
3.1.5. SEM Analysis
3.2. Catalytic Deoxygenation of the HO over the Parent HZSM-5, 5%La/HZSM-5, 10%La/HZSM-5, and 15%La/HZSM-5 Catalysts
3.2.1. Conversion of the Algal (HO)
3.2.2. Chemical Composition Group
3.2.3. The Distribution of Carbon Numbers
3.2.4. Outstanding Bio-Based Chemical Products
3.2.5. Liquid Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nuhma, M.J.; Alias, H.; Tahir, M.; Jazie, A.A. Microalgae biomass conversion into biofuel using modified HZSM-5 zeolite catalyst: A review. Mater. Today Proc. 2021, 42, 2308–2313. [Google Scholar] [CrossRef]
- Pancha, I.; Takaya, K.; Tanaka, K.; Imamura, S. The unicellular red alga Cyanidioschyzon merolae, an excellent model organism for elucidating fundamental molecular mechanisms and their applications in biofuel production. Plants 2021, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Czerwińska, J.; Lewandowski, A.; Lewandowska, W.; Sielski, J.; Dzikuć, M.; Wróbel, M.; Jewiarz, M.; et al. Sustainable drying and torrefaction processes of miscanthus for use as a pelletized solid biofuel and biocarbon-carrier for fertilizers. Molecules 2021, 26, 1014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, J.; Fa, Y. Overcoming the biological contamination in microalgae and cyanobacteria mass cultivations for photosynthetic biofuel production. Molecules 2020, 25, 5220. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A.; Estevez, R. Acetone prospect as an additive to allow the use of castor and sunflower oils as drop-in biofuels in diesel/acetone/vegetable oil triple blends for application in diesel engines. Molecules 2020, 25, 2935. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [Green Version]
- Nuhma, M.J.; Alias, H.; Jazie, A.A.; Tahir, M. Role of Microalgae as a Source for Biofuel Production in the Future: A Short Review. Bull. Chem. React. Eng. Catal. 2021, 16, 396–412. [Google Scholar] [CrossRef]
- Sousa, F.P.; Silva, L.N.; de Rezende, D.B.; de Oliveira, L.C.A.; Pasa, V.M. Simultaneous deoxygenation, cracking and isomerization of palm kernel oil and palm olein over beta zeolite to produce biogasoline, green diesel and biojet-fuel. Fuel 2018, 223, 149–156. [Google Scholar] [CrossRef]
- Meller, E.; Green, U.; Aizenshtat, Z.; Sasson, Y. Catalytic deoxygenation of castor oil over Pd/C for the production of cost effective biofuel. Fuel 2014, 133, 89–95. [Google Scholar]
- Peng, B.; Yao, Y.; Zhao, C.; Lercher, J.A. Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew. Chem. Int. Ed. 2012, 51, 2072–2075. [Google Scholar] [CrossRef]
- Peng, B.; Yuan, X.; Zhao, C.; Lercher, J.A. Stabilizing catalytic pathways via redundancy: Selective reduction of microalgae oil to alkanes. J. Am. Chem. Soc. 2012, 134, 9400–9405. [Google Scholar] [CrossRef]
- Duongbia, N.; Kannari, N.; Sato, K.; Takarada, T.; Chaiklangmuang, S. Production of bio-based chemicals from palmitic acid by catalytic hydrotreating over low-cost Ni/LY char and limonite catalysts. Alex. Eng. J. 2022, 61, 3105–3124. [Google Scholar] [CrossRef]
- Snåre, M.; Kubičková, I.; Mäki-Arvela, P.; Chichova, D.; Eränen, K.; Murzin, D.Y. Catalytic deoxygenation of unsaturated renewable feedstocks for production of diesel fuel hydrocarbons. Fuel 2008, 87, 933–945. [Google Scholar] [CrossRef]
- Shimada, I.; Kato, S.; Hirazawa, N.; Nakamura, Y.; Ohta, H.; Suzuki, K.; Takatsuka, T. Deoxygenation of triglycerides by catalytic cracking with enhanced hydrogen transfer activity. Ind. Eng. Chem. Res. 2017, 56, 75–86. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C. Development of a bimetallic Pd-Ni/HZSM-5 catalyst for the tandem limonene dehydrogenation and fatty acid deoxygenation to alkanes and arenes for use as biojet fuel. ACS Catal. 2016, 6, 4512–4525. [Google Scholar] [CrossRef]
- Na, J.-G.; Yi, B.E.; Kim, J.N.; Yi, K.B.; Park, S.; Park, J.; Kim, J.; Ko, C.H. Hydrocarbon production from decarboxylation of fatty acid without hydrogen. Catal. Today 2010, 156, 44–48. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J. Review of heterogeneous catalysts for catalytically upgrading vegetable oils into hydrocarbon biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.K.; Bhatia, S. The current status and perspectives of biofuel production via catalytic cracking of edible and non-edible oils. Energy 2010, 35, 111–119. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review. Appl. Catal. A Gen. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Yang, C.; Qiu, Q.; Yan, Q.; Li, R.; Wang, B.; Wu, J.; Ding, Y. Recent developments in commercial processes for refining bio-feedstocks to renewable diesel. BioEnergy Res. 2018, 11, 689–702. [Google Scholar] [CrossRef]
- Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; Isha, R.; Hamid, M.K.A.; Hasbullah, H.; Ali, R.R. Catalytic upgrading of sugarcane bagasse pyrolysis vapours over rare earth metal (Ce) loaded HZSM-5: Effect of catalyst to biomass ratio on the organic compounds in pyrolysis oil. Appl. Energy 2018, 220, 787–799. [Google Scholar] [CrossRef]
- Zakaria, Z.Y.; Linnekoski, J.; Amin, N.S. Catalyst screening for conversion of glycerol to light olefins. Chem. Eng. J. 2012, 207, 803–813. [Google Scholar] [CrossRef]
- Ahmad, M.; Farhana, R.; Raman, A.A.A.; Bhargava, S.K. Synthesis and activity evaluation of heterometallic nano oxides integrated ZSM-5 catalysts for palm oil cracking to produce biogasoline. Energy Convers. Manag. 2016, 119, 352–360. [Google Scholar] [CrossRef]
- Botas, J.; Serrano, D.; García, A.; de Vicente, J.; Ramos, R. Catalytic conversion of rapeseed oil into raw chemicals and fuels over Ni-and Mo-modified nanocrystalline ZSM-5 zeolite. Catal. Today 2012, 195, 59–70. [Google Scholar] [CrossRef]
- Isha, R.; Williams, P. Experimental design methodology for optimising catalytic performance of Ni/Ce/α-Al2O3 catalyst for methane steam reforming. J. Energy Inst. 2012, 85, 22–28. [Google Scholar] [CrossRef]
- Han, W.; Tang, Z.; Zhang, P.; Lu, G. Study of one step synthesis of rare earth zeolite (Ln–ZSM-5) and application for low temperature co catalytic oxidation. Catal. Surv. Asia 2013, 17, 147–155. [Google Scholar] [CrossRef]
- Shackleford, A.; Masak, T.; Fu, Q.; Smith, G.M.; Yilmaz, B.; BASF Corporation; Culp, R.D.; Gawecki, P. An alternative to rare earth elements in FCC catalysts–the use of Phinesse at Shell Sarnia. Hydrog. Eng. 2015, 20, 50–56. [Google Scholar]
- Doronin, V.; Sorokina, T.; Lipin, P.; Potapenko, O.; Korotkova, N.; Gordenko, V. Development and introduction of zeolite containing catalysts for cracking with controlled contents of rare earth elements. Catal. Ind. 2015, 7, 12–16. [Google Scholar] [CrossRef]
- Sun, L.; Guo, X.; Xiong, G.; Wang, X. Ethylation of coking benzene with ethanol over nano-sized ZSM-5 zeolites: Effects of rare earth oxides on catalyst stability. Catal. Commun. 2012, 25, 18–21. [Google Scholar] [CrossRef]
- Ouyang, J.; Kong, F.; Su, G.; Hu, Y.; Song, Q. Catalytic conversion of bio-ethanol to ethylene over La-modified HZSM-5 catalysts in a bioreactor. Catal. Lett. 2009, 132, 64–74. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, X.; Bai, T.; Zhang, Q.; Tao, L.; Qi, M.; Duan, C.; Zhang, L. Coupling conversion of methanol and C4 hydrocarbon to propylene on La-modified HZSM-5 zeolite catalysts. Ind. Eng. Chem. Res. 2012, 51, 13589–13598. [Google Scholar] [CrossRef]
- Khezri, H.; Izadbakhsh, A.; Izadpanah, A.A. Promotion of the performance of La, Ce and Ca impregnated HZSM-5 nanoparticles in the MTO reaction. Fuel Process. Technol. 2020, 199, 106253. [Google Scholar] [CrossRef]
- Liu, J.; He, D.; Chen, D.; Hao, H.; Yu, J.; Lu, J.; Liu, F.; Liu, P.; Zhao, Y.; Luo, Y. Promotional effects of rare-earth (La, Ce and Pr) modification over HZSM-5 for methyl mercaptan catalytic decomposition. J. Taiwan Inst. Chem. Eng. 2017, 80, 262–268. [Google Scholar] [CrossRef]
- Lu, J.; Hao, H.; Zhang, L.; Xu, Z.; Zhong, L.; Zhao, Y.; He, D.; Liu, J.; Chen, D.; Pu, H.; et al. The investigation of the role of basic lanthanum (La) species on the improvement of catalytic activity and stability of HZSM-5 material for eliminating methanethiol-(CH3SH). Appl. Catal. B Environ. 2018, 237, 185–197. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Kinetic modeling of catalytic cracking of C4 alkanes over La/HZSM-5 catalysts in light olefin production. J. Anal. Appl. Pyrolysis 2015, 115, 242–254. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Peng, Y.; You, T.; Hu, X. Catalytic pyrolysis kinetics behavior of Chlorella pyrenoidosa with thermal gravimetric analysis. J. Renew. Sustain. Energy 2017, 9, 063105. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Shao, S.; Dong, L.; Zhang, J.; Hu, C.; Cai, Y. Catalytic upgrading of pyrolysis vapor from rape straw in a vacuum pyrolysis system over La/HZSM-5 with hierarchical structure. Bioresour. Technol. 2018, 259, 191–197. [Google Scholar] [CrossRef]
- Zhu, L.; Fan, M.; Wang, Y.; Wang, S.; He, Y.; Li, Q. Selective conversion of furans to p-xylene with surface-modified zeolites. J. Chem. Technol. Biotechnol. 2019, 94, 2876–2887. [Google Scholar] [CrossRef]
- Browne, B.; Gibbs, R.; McLeod, J.; Parker, M.; Schwanda, W.; Warren, K. Oil extraction from microalgae. Algae Oil Extr. Capstone 2009, 2010. [Google Scholar]
- Al-lwayzy, S.H.; Yusaf, T.; Al-Juboori, R.A. Biofuels from the fresh water microalgae Chlorella vulgaris (FWM-CV) for diesel engines. Energies 2014, 7, 1829–1851. [Google Scholar] [CrossRef] [Green Version]
- Dueso, C.; Abad, A.; García-Labiano, F.; Luis, F.; Gayán, P.; Adánez, J.; Lyngfelt, A. Reactivity of a NiO/Al2O3 oxygen carrier prepared by impregnation for chemical-looping combustion. Fuel 2010, 89, 3399–3409. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.; Shiue, D.W. Reduction of nickel-alumina catalysts. Ind. Eng. Chem. Res. 1988, 27, 429–434. [Google Scholar] [CrossRef]
- Ooi, X.Y.; Oi, L.E.; Choo, M.Y.; Ong, H.C.; Lee, H.V.; Show, P.L.; Lin, Y.C.; Juan, J.C. Efficient deoxygenation of triglycerides to hydrocarbon-biofuel over mesoporous Al2O3-TiO2 catalyst. Fuel Process. Technol. 2019, 194, 106120. [Google Scholar] [CrossRef]
- Xin, H.; Guo, K.; Li, D.; Yang, H.; Hu, C. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B Environ. 2016, 187, 375–385. [Google Scholar] [CrossRef]
- Katikaneni, S.P.; Adjaye, J.D.; Bakhshi, N.N. Studies on the catalytic conversion of canola oil to hydrocarbons: Influence of hybrid catalysts and steam. Energy Fuels 1995, 9, 599–609. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Saraeian, A.; Shanks, B.H.; Mentzel, U.V.; Jensen, P.A.; Henriksen, U.B.; Ahrenfeldt, J.; Jensen, A.D. Performance-screening of metal-impregnated industrial HZSM-5/γ-Al2O3 extrudates for deoxygenation and hydrodeoxygenation of fast pyrolysis vapors. J. Anal. Appl. Pyrolysis 2020, 150, 104892. [Google Scholar] [CrossRef]
- Sheng, C.; Azevedo, J. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Oh, S.; Kim, U.-J.; Choi, I.-G.; Choi, J.W. Solvent effects on improvement of fuel properties during hydrodeoxygenation process of bio-oil in the presence of Pt/C. Energy 2016, 113, 116–123. [Google Scholar] [CrossRef]
- Treacy, M.M.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Shen, K.; Qian, W.; Wang, N.; Su, C.; Wei, F. Centrifugation-free and high yield synthesis of nanosized H-ZSM-5 and its structure-guided aromatization of methanol to 1, 2, 4-trimethylbenzene. J. Mater. Chem. A 2014, 2, 19797–19808. [Google Scholar] [CrossRef]
- Shaikh, I.R.; Shaikh, R.A.; Shaikh, A.A.; War, J.A.; Hangirgekar, S.P.; Shaikh, A.L.; Shaikh, P.R.; Shaikh, R.R. H-ZSM-5 zeolite synthesis by sourcing silica from the wheat husk ash: Characterization and application as a versatile heterogeneous catalyst in organic transformations including some multicomponent reactions. J. Catal. 2015, 2015, 805714. [Google Scholar] [CrossRef]
- Murata, K.; Liu, Y.; Inaba, M.; Takahara, I. Production of synthetic diesel by hydrotreatment of jatropha oils using Pt− Re/H-ZSM-5 catalyst. Energy Fuels 2010, 24, 2404–2409. [Google Scholar] [CrossRef]
- Romero, M.D.; Calles, J.A.; Rodríguez, A. Influence of the preparation method and metal precursor compound on the bifunctional Ni/HZSM-5 catalysts. Ind. Eng. Chem. Res. 1997, 36, 3533–3540. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.Y.; Jiang, T.; Wang, T.J.; Ma, L.L.; Zhang, Q.; Zhang, X.H. Hydrodeoxygenation of vegetable oils to liquid alkane fuels over Ni/HZSM-5 catalysts: Methyl hexadecanoate as the model compound. Catal. Commun. 2012, 20, 80–84. [Google Scholar] [CrossRef]
- Li, B.; Li, S.; Li, N.; Chen, H.; Zhang, W.; Bao, X.; Lin, B. Structure and acidity of Mo/ZSM-5 synthesized by solid state reaction for methane dehydrogenation and aromatization. Microporous Mesoporous Mater. 2006, 88, 244–253. [Google Scholar] [CrossRef]
- Dumitriu, D.; Bârjega, R.; Frunza, L.; Macovei, D.; Hu, T.; Xie, Y.; Pârvulescu, V.I.; Kaliaguine, S. BiOx clusters occluded in a ZSM-5 matrix: Preparation, characterization, and catalytic behavior in liquid-phase oxidation of hydrocarbons. J. Catal. 2003, 219, 337–351. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. HZSM-5 zeolites with different SiO2/Al2O3 ratios. Characterization and NH3 desorption kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Hardenberg, T.; Mertens, L.; Mesman, P.; Muller, H.; Nicolaides, C. A catalytic method for the quantitative evaluation of crystallinites of ZSM-5 zeolite preparations. Zeolites 1992, 12, 685–689. [Google Scholar] [CrossRef]
- Zhu, L.; Jin, F.; Fan, M.; Liu, J.; Chang, R.; Jia, Q.; Tang, C.; Li, Q. Bio-oil as a potential biomass-derived renewable raw material for bio-phenol production. Chem. Eng. Technol. 2018, 41, 1027–1034. [Google Scholar] [CrossRef]
- Wu, X.-P.; Fan, M.-H.; Li, Q.-X. Production of benzene from lignin through current enhanced catalytic conversion. Chin. J. Chem. Phys. 2017, 30, 479. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Dou, B.; Lv, G.; Wang, C.; Hao, Q.; Hui, K. Cerium doped copper/ZSM-5 catalysts used for the selective catalytic reduction of nitrogen oxide with ammonia. Chem. Eng. J. 2015, 270, 549–556. [Google Scholar] [CrossRef]
- Zhou, W.-F.; Chen, L.; Xie, J.; Au, C.-T.; Yin, S.-F. Efficient synthesis of p-chlorobenzaldehyde through liquid-phase oxidation of p-chlorotoluene using manganese-containing ZSM-5 as catalyst. RSC Adv. 2015, 5, 74162–74169. [Google Scholar] [CrossRef]
- Xie, J.; Chen, L.; Wang, W.-H.; Wang, P.; Au, C.-T.; Yin, S.-F. Direct dual-template synthesis of HZSM-5 zeolite for enhanced p-xylene selectivity in toluene methylation with CH 3 Br. Catal. Sci. Technol. 2017, 7, 1211–1216. [Google Scholar] [CrossRef]
- Zhou, G.; Li, J.; Yu, Y.; Li, X.; Wang, Y.; Wang, W.; Komarneni, S. Optimizing the distribution of aromatic products from catalytic fast pyrolysis of cellulose by ZSM-5 modification with boron and co-feeding of low-density polyethylene. Appl. Catal. A Gen. 2014, 487, 45–53. [Google Scholar] [CrossRef]
- Jiang, P.; Wu, X.; Zhu, L.; Jin, F.; Liu, J.; Xia, T.; Wang, T.; Li, Q. Production of jet fuel range paraffins by low temperature polymerization of gaseous light olefins using ionic liquid. Energy Convers. Manag. 2016, 120, 338–345. [Google Scholar] [CrossRef]
- Katada, N.; Igi, H.; Kim, J.-H.; Niwa, M. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium. J. Phys. Chem. B 1997, 101, 5969–5977. [Google Scholar] [CrossRef]
- Rodríguez-González, L.; Hermes, F.; Bertmer, M.; Rodríguez-Castellón, E.; Jiménez-López, A.; Simon, U. The acid properties of H-ZSM-5 as studied by NH3-TPD and 27Al-MAS-NMR spectroscopy. Appl. Catal. A Gen. 2007, 328, 174–182. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.; Moulijn, J.A.; Pérez-Ramírez, J. Mechanism of hierarchical porosity development in MFI zeolites by desilication: The role of aluminium as a pore-directing agent. Chem.–A Eur. J. 2005, 11, 4983–4994. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Sápi, A.; Kashaboina, U.; Ábrahámné, K.B.; Gómez-Pérez, J.F.; Szenti, I.; Halasi, G.; Kiss, J.; Nagy, B.; Varga, T.; Kukovecz, Á.; et al. Synergetic of Pt nanoparticles and H-ZSM-5 zeolites for efficient CO2 activation: Role of interfacial sites in high activity. Front. Mater. 2019, 6, 127. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; Callén, M.; García, T. Catalytic upgrading of biomass derived pyrolysis vapors over metal-loaded ZSM-5 zeolites: Effect of different metal cations on the bio-oil final properties. Microporous Mesoporous Mater. 2015, 209, 189–196. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Wan, K.; Liu, Q. Synthesis, characterization and catalytic properties of nitrogen-incorporated ZSM-5 molecular sieves with bimodal pores. Appl. Catal. A Gen. 2004, 258, 55–61. [Google Scholar] [CrossRef]
- Wakui, K.I.; Sato, K.; Sawada, G.; Shiozawa, K.; Matano, K.; Suzuki, K.; Hayakawa, T.; Murata, K.; Yoshimura, Y.; Mizukami, F. Catalytic cracking of n-butane over rate earth-loaded HZSM-5 catalysts; Kidoruishushoku HZSM-5 shokubai niyoru n-butan no sesshoku bunkai hanno. Sekiyu Gakkai-Shi 1999, 42, 9340. [Google Scholar] [CrossRef]
- Xiaoning, W.; Zhen, Z.; Chunming, X.; Aijun, D.; Li, Z.; Guiyuan, J. Effects of light rare earth on acidity and catalytic performance of HZSM-5 zeolite for catalytic cracking of butane to light olefins. J. Rare Earths 2007, 25, 321–328. [Google Scholar] [CrossRef]
- Zheng, L.; Xuan, D.; Guo, J.; Lou, H.; Zheng, X. Non-oxidative aromatization of CH4-C3H8 over La-promoted Zn/HZSM-5 catalysts. J. Nat. Gas Chem. 2006, 15, 52–57. [Google Scholar] [CrossRef]
- Sousa-Aguiar, E.F.; Trigueiro, F.E.; Zotin, F.M.Z. The role of rare earth elements in zeolites and cracking catalysts. Catal. Today 2013, 218, 115–122. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater. 2015, 203, 41–53. [Google Scholar] [CrossRef]
- Bruno, J.E.; Dooley, K.M. Regeneration of a supported Nafion® catalyst for the double-bond isomerization of octadecenes. Appl. Catal. A Gen. 2016, 526, 70–76. [Google Scholar] [CrossRef]
- Kolesnichenko, N.; Kolesnikova, E.; Kitaev, L.; Biryukova, E.; Trukhmanova, N.; Khadzhiev, S. Zeolite catalysts modified with zirconium and sulfur compounds in the conversion of dimethyl ether to lower olefins. Pet. Chem. 2012, 52, 155–160. [Google Scholar] [CrossRef]
- Kham-or, P.; Suwannasom, P.; Ruangviriyachai, C. Effect of agglomerated NiMo HZSM-5 catalyst for the hydrocracking reaction of Jatropha curcas oil. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3694–3701. [Google Scholar] [CrossRef]
- Tishchenko, I.Y.; Ilchenko, O.; Kuzema, P. TGA-DSC-MS analysis of silicon carbide and of its carbon-silica precursor. Chem. Phys. Technol. Surf. 2015, 6, 216–223. [Google Scholar]
- Palizdar, A.; Sadrameli, S. Catalytic upgrading of beech wood pyrolysis oil over iron-and zinc-promoted hierarchical MFI zeolites. Fuel 2020, 264, 116813. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Tailoring the mesopore structure of HZSM-5 to control product distribution in the conversion of propanal. J. Catal. 2010, 271, 88–98. [Google Scholar] [CrossRef]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of triglycerides to hydrocarbons over supported metal catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]
- Snåre, M.; Kubičkova, I.; Mäki-Arvela, P.; Eränen, K.; Murzin, D.Y. Heterogeneous catalytic deoxygenation of stearic acid for production of biodiesel. Ind. Eng. Chem. Res. 2006, 45, 5708–5715. [Google Scholar] [CrossRef]
- Silva, L.N.; Fortes, I.C.; de Sousa, F.P.; Pasa, V.M. Biokerosene and green diesel from macauba oils via catalytic deoxygenation over Pd/C. Fuel 2016, 164, 329–338. [Google Scholar] [CrossRef]
- Li, C.; Ma, J.; Xiao, Z.; Hector, S.B.; Liu, R.; Zuo, S.; Xie, X.; Zhang, A.; Wu, H.; Liu, Q. Catalytic cracking of Swida wilsoniana oil for hydrocarbon biofuel over Cu-modified ZSM-5 zeolite. Fuel 2018, 218, 59–66. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Huang, Y.; Yu, Y.; Julson, J. Catalytic cracking of camelina oil for hydrocarbon biofuel over ZSM-5-Zn catalyst. Fuel Process. Technol. 2015, 139, 117–126. [Google Scholar] [CrossRef]
- Morgan, T.; Santillan-Jimenez, E.; Harman-Ware, A.E.; Ji, Y.; Grubb, D.; Crocker, M. Catalytic deoxygenation of triglycerides to hydrocarbons over supported nickel catalysts. Chem. Eng. J. 2012, 189, 346–355. [Google Scholar] [CrossRef]
- Hollak, S.A.; Bitter, J.H.; van Haveren, J.; de Jong, K.P.; van Es, D.S. Selective deoxygenation of stearic acid via an anhydride pathway. RSC Adv. 2012, 2, 9387–9391. [Google Scholar] [CrossRef]
- Li, J.; Qin, G.; Tang, X.; Xiang, C. Fe/HZSM-5 catalyzed liquefaction of cellulose assisted by glycerol. Catal. Commun. 2021, 151, 106268. [Google Scholar] [CrossRef]
- Rozmyslowicz, B. Deoxygenation of Fatty Acids for Production of Fuels and Chemicals. Ph.D Thesis, Åbo Akademi University, Turku, Finland, 2014. [Google Scholar]
- Zheng, Z.; Lei, T.; Wang, J.; Wei, Y.; Liu, X.; Yu, F.; Ji, J. Catalytic cracking of soybean oil for biofuel over γ-Al2O3/CaO composite catalyst. J. Braz. Chem. Soc. 2019, 30, 359–370. [Google Scholar] [CrossRef]
- Jafarian, S.; Tavasoli, A.; Nikkhah, H. Catalytic hydrotreating of pyro-oil derived from green microalgae spirulina the (Arthrospira) plantensis over NiMo catalysts impregnated over a novel hybrid support. Int. J. Hydrogen Energy 2019, 44, 19855–19867. [Google Scholar] [CrossRef]
- Oh, S.; Choi, H.S.; Choi, I.-G.; Choi, J.W. Evaluation of hydrodeoxygenation reactivity of pyrolysis bio-oil with various Ni-based catalysts for improvement of fuel properties. RSC Adv. 2017, 7, 15116–15126. [Google Scholar] [CrossRef]
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014, 16, 491–515. [Google Scholar] [CrossRef]












| No. | Catalyst Name | Relative Crystallinity (%) |
|---|---|---|
| 1 | HZSM-5 | 100 |
| 2 | 5%Ce/HZSM-5 | 115 |
| 3 | 10%Ce/HZSM-5 | 78 |
| 4 | 15%Ce/HZSM-5 | 57 |
| No | Catalyst | SBET (m2/g) | Smicro (m2/g) | Sextern (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Average Particle Size (nm) |
|---|---|---|---|---|---|---|---|
| 1 | HZSM-5 | 338 | 195 | 143 | 0.22 | 0.100 | 17 |
| 2 | 5%Ce/HZSM-5 | 308 | 166 | 142 | 0.2 | 0.085 | 19 |
| 3 | 10%Ce/HZSM-5 | 286 | 154 | 131 | 0.19 | 0.081 | 20 |
| 4 | 15%Ce/HZSM-5 | 258 | 142 | 115 | 0.17 | 0.074 | 23 |
| Catalyst | Low Peak Temperature Point (°C) (Weak Acid Peak) | High Peak Temperature Point (°C) (Strong Acid Peak) | Total Acid Amount (Total NH3 Amount mmol/g) | ||||
|---|---|---|---|---|---|---|---|
| T (°C) | TCD (V) | NH3 Amount (mmol/g) | T (°C) | TCD (V) | NH3 Amount (mmol/g) | ||
| HZSM-5 | 216 | 0.0808 | 0.526 | 439 | 0.0295 | 0.214 | 0.740 |
| 5%Ce /HZSM-5 | 208.500 | 0.0523 | 0.358 | 449 | 0.0221 | 0.178 | 0.536 |
| 10%Ce /HZSM-5 | 214 | 0.0459 | 0.340 | 420 | 0.0218 | 0.166 | 0.506 |
| 15%Ce /HZSM-5 | 212.900 | 0.0394 | 0.331 | 412.3 | 0.0201 | 0.159 | 0.490 |
| Compounds of the Algal (HO) | Molecular Formula | Content of the Compound in the Feed (HO) (wt. %) | Content (wt. %) of the Compound in the Liquid Product of the Catalytic Deoxygenation Reactions for the Algal HO as a Function of the Cerium-loading Percentage on the Parent HZSM-5 | |||
|---|---|---|---|---|---|---|
| HZSM-5 | 5%Ce/HZSM-5 | 10%Ce/HZSM-5 | 15%Ce/HZSM-5 | |||
| Hexacosane | C26H54 | 11.53 | 0 | 0 | 0 | 0 |
| 6-Octen-1-ol, 3,7-dimethyl-, formate | C11H20O2 | 1.72 | 0 | 0 | 0 | 0 |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | C19H32O2 | 15.08 | 0 | 0 | 0 | 0 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 15.32 | 5.41 | 2.8 | 1.79 | 3.78 |
| 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 27.69 | 0 | 0 | 0 | 0 |
| Di-n-octyl phthalate | C24H38O4 | 1.29 | 0 | 0 | 0 | 0 |
| Phytol | C20H40O | 21.62 | 40.85 | 40.03 | 48 | 40.14 |
| others | - | 5.71 | 0 | 0 | 0 | 0 |
| Conversion (%) of the algal HO in the catalytic deoxygenation reactions as a function of the Cerium-loading percentage on the parent HZSM-5 | 94.58 | 97.19 | 98.20 | 96.21 | ||
| Compound | Molecular Formula | Algal Hydrolyzed Oil (HO) | HZSM-5 | 5%Ce/ HZSM-5 | 10%Ce/ HZSM-5 | 15%Ce/ HZSM-5 |
|---|---|---|---|---|---|---|
| ALKANE | ||||||
| Hexacosane | C26H54 | 11.53 | ||||
| Tetradecane | C14H30 | 4.78 | 4.2 | 5.21 | 5.36 | |
| Octane | C8H18 | 1.05 | ||||
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.) | C10H18 | 4.38 | ||||
| TOTAL ALKANES | 11.53 | 4.78 | 5.26 | 5.21 | 9.74 | |
| ALKENS | ||||||
| 5-Ethyl-1-nonene | C11H22 | 17.04 | 17.75 | 20.65 | 4.83 | |
| 1-Undecene, 8-methyl- | C12H24 | 4.13 | 5.1 | |||
| 2-Hexadecene, 2,6,10,14-tetramethyl- | C20H40 | 2.49 | ||||
| TOTAL ALKENS | 0 | 17.04 | 17.75 | 24.78 | 12.43 | |
| ESTERS | ||||||
| 6-Octen-1-ol, 3,7-dimethyl-, formate | C11H20O2 | 1.72 | ||||
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | C19H32O2 | 15.08 | ||||
| Hexadecanoic acid, methyl ester | C17H34O2 | 15.32 | 5.41 | 2.8 | 1.79 | 3.78 |
| Carbonic acid, butyl undec-10-enyl ester | C16H30O3 | 1.88 | ||||
| 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 27.69 | ||||
| Di-n-octyl phthalate | C24H38O4 | 1.29 | ||||
| trans-13-Octadecenoic acid, methyl ester | C19H36O2 | 5.34 | ||||
| 2-(Prop-2-enoyloxy)tetradecane | C17H32O2 | 14.19 | ||||
| Oxalic acid, hexyl octadecyl ester | C26H50O4 | 1.1 | ||||
| Decyl oleate | C28H54O2 | 1.19 | ||||
| 6-Octadecenoic acid, methyl ester, (Z)- | C19H36O2 | 3.65 | ||||
| 11-Octadecenoic acid, methyl ester | C19H36O2 | 5.55 | ||||
| TOTAL ESTERS | 61.12 | 12.64 | 17 | 7.74 | 9.34 | |
| ETHERS | ||||||
| Disparlure | C19H38O | 1.46 | 0.53 | |||
| Tetrahydropyran 12-tetradecyn-1-ol ether | C19H34O2 | 1.37 | ||||
| Oxirane, tridecyl- | C15H30O | 2.08 | 1.16 | 0.71 | 1.6 | |
| 2H-Pyran, 2-(7-dodecynyloxy)tetrahydro- | C17H30O2 | 1.38 | ||||
| TOTAL ETHERS | 0 | 4.93 | 1.162 | 1.25 | 2.99 | |
| ALDEHYDES | ||||||
| Tetradecanal | C14H28O | 6.47 | 0.79 | |||
| 13-Octadecenal, (Z)- | C18H34O | 0.38 | ||||
| cis-9-Hexadecenal | C16H30O | 1.99 | ||||
| 2-Heptadecenal | C17H32O | 1.21 | ||||
| TOTAL ALDEHYDES | 0 | 6.47 | 1.17 | 1.99 | 1.21 | |
| KETONES | ||||||
| 2-Pentadecanone, 6,10,14-trimethyl | C18H36O | 2.39 | ||||
| 9-(Tetrahydropyran-2-yloxy)-4,6-dioxatricyclo[5.3.1.0(3,8)]undecan-5-one | C14H20O5 | 1.29 | ||||
| 4,7,7-Trimethyl-5-(tetrahydropyran-2-yloxy)-bicyclo[2.2.1]heptan-2-one | C15H24O3 | 1.32 | ||||
| 1-Cyclohexene, 1,3,3-trimethyl-2-(1-methylbut-1-en-3-on-1-yl)- | C14H22O | 1.13 | ||||
| TOTAL KETONES | 0 | 2.394 | 1.29 | 1.32 | 1.13 | |
| ALCOHOLS | ||||||
| 1-Dodecanol, 3,7,11-trimethyl- | C15H32O | 8.69 | 4.11 | 17.28 | ||
| 2-Propylcyclohexanol | C9H18O | 2.99 | ||||
| Phytol | C20H40O | 21.62 | 40.85 | 40.03 | 48 | 40.14 |
| Behenic alcohol | C22H46O | 1.13 | ||||
| 2-Norpinanol, 3,6,6-trimethyl- | C10H18O | 5.20 | ||||
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 4.29 | ||||
| TOTAL ALCOHOLS | 21.62 | 49.54 | 53.48 | 52.3 | 57.43 | |
| TOTAL Areas (%) | 94.28 | 97.81 | 97.14 | 94.62 | 94.3 | |
| Others Areas (%) = 100-Total Areas (%) | 5.71 | 2.18 | 2.85 | 5.37 | 5.69 | |
| Hydrocarbon Compound | Molecular Formula | Hydrolyzed Oil (HO) | HZSM-5 | 5%Ce/ HZSM-5 | 10%Ce/ HZSM-5 | 15%Ce/ HZSM-5 |
|---|---|---|---|---|---|---|
| Hexacosane | C26H54 | 11.53 | ||||
| Tetradecane | C14H30 | 4.78 | 4.2 | 5.21 | 5.36 | |
| Octane | C8H18 | 1.05 | ||||
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.) | C10H18 | 4.38 | ||||
| 5-Ethyl-1-nonene | C11H22 | 17.04 | 17.75 | 20.65 | 4.83 | |
| 1-Undecene, 8-methyl- | C12H24 | 4.13 | 5.1 | |||
| 2-Hexadecene, 2,6,10,14-tetramethyl- | C20H40 | 2.49 | ||||
| The total yield of the hydrocarbon compounds | 11.53 | 21.83 | 23.02 | 30 | 22.17 | |
| Alcohol Compound | Molecular formula | Algal HO | HZSM-5 | 5%Ce /HZSM-5 | 10%Ce /HZSM-5 | 15%Ce /HZSM-5 |
| 1-Dodecanol, 3,7,11-trimethyl- | C15H32O | 8.69 | 4.11 | 17.28 | ||
| 2-Propylcyclohexanol | C9H18O | 2.99 | ||||
| Phytol | C20H40O | 21.62 | 40.85 | 40.03 | 48 | 40.14 |
| Behenic alcohol | C22H46O | 1.13 | ||||
| 2-Norpinanol, 3,6,6-trimethyl- | C10H18O | 5.2 | ||||
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 4.29 | ||||
| The total yield of the alcohol compounds | 21.62 | 49.54 | 53.48 | 52.303 | 57.43 | |
| Reactant | Catalyst | Reactant/Catalyst Ratio | Reactant/Solvent | Reactor Type | Pressure (bar), Gas | Temperature (°C) | Time (h) | Conversion (%) | Observations | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| palm kernel oil | HBeta zeolite | 10/1.5 | - | B.R | 10 bar H2 | 350 | 5 | - | The total yield of hydrocarbons = 82 ± 3% | [8] |
| Hydrolyzed palm kernel oil | HBeta zeolite | 10/1.5 | - | B.R | 10 bar H2 | 350 | 5 | - | The total yield of hydrocarbons = 24 ± 9% | [8] |
| Olein oil | HBeta zeolite | 10/1.5 | - | B.R | 10 bar H2 | 350 | 5 | - | The total yield of hydrocarbons = 43 ± 3% | [8] |
| Hydrolyzed olein oil | HBeta zeolite | 10/1.5 | - | B.R | 10 bar H2 | 350 | 5 | - | The total yield of hydrocarbons = 98 ± 4% | [8] |
| Hydrolyzed Macauba oil | HBeta zeolite | 10/1 | - | B.R | 10 bar H2 | 350 | 5 | - | The total yield of hydrocarbons = 30% | [8] |
| Hydrolyzed castor oil | 5% Pd/C | 1/0.1 | 1 g Hydrolyzed castor oil/30 mL n-hexane | B.R | 25 bar H2 | 310 | 7 | - | The total yield of hydrocarbons = 57% | [9] |
| Hydrolyzed castor oil | 5% Pd/C | 1/0.1 | 1 g Hydrolyzed castor oil/30 mL n-dodecane | B.R | 25 bar H2 | 310 | 7 | - | The total yield of hydrocarbons = 39.6% | [9] |
| Hydrolyzed castor oil | 5% Pd/C | 1/0.1 | 1 g Hydrolyzed castor oil/30 mL n-hexane | B.R | 25 bar H2 | 300 | 7 | - | The total yield of hydrocarbons = 40% | [9] |
| Hydrolyzed castor oil | 5% Pd/C | 1/0.1 | 1 g Hydrolyzed castor oil/30 mL n-hexane | B.R | 25 bar H2 | 340 | 7 | - | The total yield of hydrocarbons ~96% | [9] |
| Stearic acid | 10%Ni/HZSM-5 (Si/Al = 40) | 1/0.2 | 1 g stearic acid/ 100 mL dodecane | B.R | 40 bar H2 | 260 | 8 | Total selectivity of hydrocarbons ~56% | [10] | |
| Microalgae oil | 10%Ni/HBeta (Si/Al =180) | 1/0.2 | 1 g Microalgae oil/100 mL dodecane | B.R | 40 bar H2 | 260 | 6 | The total yield of hydrocarbons = 70% | [10] | |
| Crude oil of microalgae | 10%Ni/ ZrO2 | 1/0.5 | - | B.R | 40 bar H2 | 270 | 6 | - | The total yield of hydrocarbons = 72% | [11] |
| Crude oil of microalgae | 10%Ni/ ZrO2 | 1/0.5 | - | B.R | 40 bar H2 | 270 | 4 | - | The total yield of hydrocarbons = 61% | [11] |
| Palmitic acid | Ni/LY char | 1/1 | 1 g Palmtic acid/ 10 g hexane | B.R | 30 bar H2 | 300 | 5 | 31.41 | The total yield of hydrocarbons = 12.75% | [12] |
| Palmitic acid | Ni/LY char | 1/1 | 1 g Palmtic acid/ 10 g acetone | B.R | 30 bar H2 | 300 | 5 | 67 | The total yield of hydrocarbons = 12.49% | [12] |
| Methyl oleate | 5%Pd/C | 0.83 mol/L/ 1 g of catalyst | - | Semi-batch | 15 bar H2 | 300 | 6 | 96 | Total selectivity of hydrocarbons = 29% | [13] |
| Methyl oleate | 5%Pd/C | 0.83 mol/L/ 1 g of catalyst | - | Semi-batch | 15 bar Ar | 300 | 6 | 44 | Total selectivity of hydrocarbons = 17% | [13] |
| Soybean oil | 20%Ni/ Al2O3 | 50/0.55 | - | B.R | 7 bar N2 | 350 | 4 | 74 | The total yield of hydrocarbons = 79.5% | [86] |
| Stearic acid | Pd/ Al2O3 | 1 | - | B.R | 7 bar N2 | 350 | 6 | 43 | Total selectivity of hydrocarbons = 35% | [87] |
| Cellulose and glycerol | HZSM-5 (Si/Al = 36) | cellulose:glycerol: catalyst = 1:0.05:0.004 | 100 g of n-heptane | B.R | - | 350 | 0.5 | - | The total yield of hydrocarbons = 21% | [88] |
| Cellulose and glycerol | 5%Fe/ HZSM-5 (Si/Al = 36) | cellulose:glycerol: catalyst = 1:0.05:0.004 | 100 g of n-heptane | B.R | - | 350 | 0.5 | - | The total yield of hydrocarbons = 38% | [88] |
| Lauric acid | 5%Pd/C | 1/0.1 | 1 g of acid/ 100 mL of hexadecane | S.B.R | 20 bar Ar | 300 | 6 | - | The total yield of hydrocarbons = 38 | [89] |
| Lauric acid | 5%Pd/C | 1/0.1 | 1 g of acid/ 100 mL of hexadecane | S.B.R | 20 bar Ar | 300 | 3 | - | The total yield of hydrocarbons = 28 | [89] |
| Algal HO | HZSM-5 (Si/Al = 30) | 1 g of algal HO/0.15 g of the catalyst | - | B.R | 7 bar N2 | 300 | 6 | 94.58 | The total yield of hydrocarbons = 21.83% | This study |
| Algal HO | 5%Ce/ HZSM-5 (Si/Al = 30) | 1 g of algal HO/0.15 g of the catalyst | - | B.R | 7 bar N2 | 300 | 6 | 97.19 | The total yield of hydrocarbons = 23.02% | This study |
| Algal HO | 10%Ce/ HZSM-5 (Si/Al = 30) | 1 g of algal HO/0.15 g of the catalyst | - | B.R | 7 bar N2 | 300 | 6 | 98.20 | The total yield of hydrocarbons = 30% | This study |
| Algal HO | 15%Ce/ HZSM-5 (Si/Al = 30) | 1 g of algal HO/0.15 g of the catalyst | - | B.R | 7 bar N2 | 300 | 6 | 96.21 | The total yield of hydrocarbons = 22.17% | This study |
| Reactant | Catalyst | Operating Conditions | Observation | Ref. |
|---|---|---|---|---|
| Palmitic acid | Limonite catalyst | The catalytic hydrotreating of the palmitic acid was conducted in a batch reactor with the weight ratio of palmitic acid:catalyst: solvent (hexane) of 1:1:10, respectively at 300 °C, 30 bar of H2. | The total yield percentage of alcohol = 51.84 and 38.35 at 5 h and 3 h respectively | [12] |
| Cellulose, and glycerol | HZSM-5 (Si/Al = 36) and 5%Fe/ HZSM-5 (Si/Al = 36) | Cellulose, glycerol, and catalyst were added to the batch reactor with weight ratios of 1:0.05:0.004 respectively, then 100 g of n-heptane was added, and the reaction temperature is 350 °C for 0.5 h | The total yield of alcohol compounds~ 26% using HZSM-5 and about ~20% using 5%Fe/HZSM-5 | [92] |
| Lauric acid | 5%Pd/C | 1 g of Lauric acid with 100 mL of hexadecane and 0.1 g of catalyst was added to the semi-batch reactor. The deoxygenation reaction temperature was performed at a temperature of 300 °C, and 6 h. | The total yield percentage of alcohol = 9% and 0% using 20 bar of H2 and 20 bar of Ar, respectively. | [93] |
| Stearic acid | 4%Ru/ TiO2 | 1 g of stearic acid with 100 mL of dodecane and 0.1 g of catalyst was added to the semi-batch reactor. The deoxygenation reaction temperature was performed at a temperature of 220 °C, and 20 bar of H2. | The total yield percentage of alcohol= 20% and 0% at 1 h and 6 h, respectively. | [93] |
| Stearic acid | 4%Re/ TiO2 | 1 g of stearic acid with 100 mL of dodecane and 0.1 g of catalyst was added to the semi-batch reactor. The deoxygenation reaction temperature was performed at a temperature of 220 °C, 6 h, and 20 bar of H2. | The total yield percentage (%) of alcohol = 81% | [93] |
| Soybean oil | 35%ɤAl2O3/CaO | The experiment was conducted under atmospheric pressure in a fixed bed reactor; 6 g of catalyst was placed in the middle of the reactor, then 24 g of soybean oil was injected with WHSV = 3.72 h−1 at 480 °C. | The total yield of alcohol compounds = 12.3% | [94] |
| Sugarcane bagasse | HZSM-5 (Si/Al = 23) and 1%Ce/ HZSM-5 (Si/Al = 23) | The catalytic pyrolysis experiment was conducted under atmospheric pressure in a fixed bed reactor. 1 g of catalyst and 2 g of Sugarcane bagasse were placed in the reactor at 500 °C. | The total yield percentage (%) of alcohol~14% and ~5% using HZSM-5(Si/Al = 23) and 1%Ce/HZSM-5(Si/Al = 23), respectively. | [21] |
| Algal HO | HZSM-5 (Si/Al = 30), 5%Ce/HZSM-5 (Si/Al = 30), 10%Ce/HZSM-5 (Si/Al = 30), and 15%Ce/HZSM-5 (Si/Al = 30) | The catalytic deoxygenation of the algal HO in the batch reactor at 300 °C, 6 h, 7 bar of initial inert N2 gas, 1 g of algal HO/0.15 g of the catalyst. | The total yield percentage (%) of alcohol = 49.54, 53.48, 52.30, and 57.43 using HZSM-5, 5%Ce/HZSM-5, 10%Ce/HZSM-5, and 15%Ce/HZSM-5, respectively. | Current study |
| NO. | Liquid Type | Element (%) | HHV (MJ/Kg) | H/C (Mole Ratio) | O/C (Mole Ratio) | DOD% | ||
|---|---|---|---|---|---|---|---|---|
| C | H | O | ||||||
| 1 | Algal hydrolyzed oil (HO) | 78.91 | 12.43 | 8.65 | 32.37 | 1.89 | 0.08 | n.a |
| 2 | Liquid product for HZSM-5 | 82.9 | 12.02 | 5.06 | 33.23 | 1.74 | 0.04 | 44.235 |
| 3 | Liquid product for 5%Ce/HZSM-5 | 80.66 | 13.34 | 5.99 | 33.48 | 1.98 | 0.05 | 32.22 |
| 4 | Liquid product for 10%Ce/HZSM-5 | 82 | 13.63 | 4.36 | 34.05 | 1.99 | 0.03 | 51.44 |
| 5 | Liquid product for 15%Ce/HZSM-5 | 81.23 | 13.61 | 5.14 | 33.82 | 2.01 | 0.04 | 42.26 |
| 6 | Crude oil [93] | 83–86 | 11–14 | ˂ 1 | 44 | 1.5–2 | ~ 0 | n.a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuhma, M.J.; Alias, H.; Tahir, M.; Jazie, A.A. Ce-Loaded HZSM-5 Composite for Catalytic Deoxygenation of Algal Hydrolyzed Oil into Hydrocarbons and Oxygenated Compounds. Molecules 2022, 27, 7251. https://doi.org/10.3390/molecules27217251
Nuhma MJ, Alias H, Tahir M, Jazie AA. Ce-Loaded HZSM-5 Composite for Catalytic Deoxygenation of Algal Hydrolyzed Oil into Hydrocarbons and Oxygenated Compounds. Molecules. 2022; 27(21):7251. https://doi.org/10.3390/molecules27217251
Chicago/Turabian StyleNuhma, Mustafa Jawad, Hajar Alias, Muhammad Tahir, and Ali A. Jazie. 2022. "Ce-Loaded HZSM-5 Composite for Catalytic Deoxygenation of Algal Hydrolyzed Oil into Hydrocarbons and Oxygenated Compounds" Molecules 27, no. 21: 7251. https://doi.org/10.3390/molecules27217251
APA StyleNuhma, M. J., Alias, H., Tahir, M., & Jazie, A. A. (2022). Ce-Loaded HZSM-5 Composite for Catalytic Deoxygenation of Algal Hydrolyzed Oil into Hydrocarbons and Oxygenated Compounds. Molecules, 27(21), 7251. https://doi.org/10.3390/molecules27217251