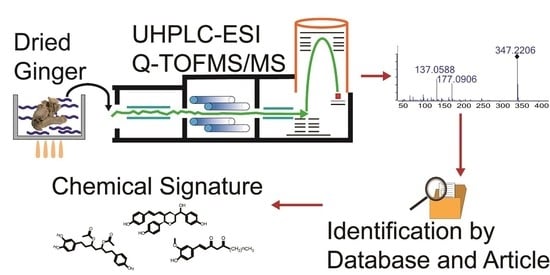
The Chemical Signatures of Water Extract of Zingiber officinale Rosc
Abstract
:1. Introduction
2. Results and Discussion
2.1. LC/MS Conditions
2.2. Establishment of Fragmentation Patterns
2.3. Gingerol-Related Compounds
2.4. Gingerdione-Related Compounds
2.5. Gingerdiol-Related Compounds
2.6. Shogaol and Paradol-Related Compounds
2.7. Diarylheptanoids
2.8. Two Amino Acids, Four Fatty Acids, and Others
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant-Material and Sample Preparation
3.3. Accurate-Mass QTOF LC/MS System
3.4. Mass Spectrometry
3.5. Building the Chemical Database of Ginger
3.6. Preparation of the Reference Standard
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-Inflammatory Effects of the Essential Oils of Ginger (Zingiber officinale Roscoe) in Experimental Rheumatoid Arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, G.; Al-Kahtani, M.A.; El-Sayed, W.M. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation 2011, 34, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.; El-Menshawy, O. Protective effects of ginger-turmeric rhizomes mixture on joint inflammation, atherogenesis, kidney dysfunction and other complications in a rat model of human rheumatoid arthritis. Int. J. Rheum. Dis. 2013, 16, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, M.N.; Gilani, A.H. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig. Dis. Sci. 2005, 50, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Walstab, J.; Kruger, D.; Stark, T.; Hofmann, T.; Demir, I.E.; Ceyhan, G.O.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol Motil. 2013, 25, 439–447-e302. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef]
- Wang, C.Z.; Qi, L.W.; Yuan, C.S. Cancer Chemoprevention Effects of Ginger and its Active Constituents: Potential for New Drug Discovery. Am. J. Chin. Med. 2015, 43, 1351–1363. [Google Scholar] [CrossRef]
- Zick, S.M.; Turgeon, D.K.; Ren, J.; Ruffin, M.T.; Wright, B.D.; Sen, A.; Djuric, Z.; Brenner, D.E. Pilot clinical study of the effects of ginger root extract on eicosanoids in colonic mucosa of subjects at increased risk for colorectal cancer. Mol. Carcinog. 2015, 54, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Marx, W.; Ried, K.; McCarthy, A.L.; Vitetta, L.; Sali, A.; McKavanagh, D.; Isenring, L. Ginger-Mechanism of action in chemotherapy-induced nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 141–146. [Google Scholar] [CrossRef]
- Murakami, A.; Hayashi, R.; Tanaka, T.; Kwon, K.H.; Ohigashi, H.; Safitri, R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: Separately and in combination. Biochem. Pharmacol. 2003, 66, 1253–1261. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohns Colitis. 2018, 12, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alinkina, E.S.; Misharina, T.A.; Fatkullina, L.D.; Burlakova, E.B. Comparison of the antiradical activity of ionol, components of fresh ginger, and its extracts. Prikl. Biokhim. Mikrobiol. 2012, 48, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Amer, A.M.; Abulifa, A.R.; Elzwawi, M.M. Ginger components as new leads for the design and development of novel multi-targeted anti-Alzheimer’s drugs: A computational investigation. Drug Des. Devel. Ther. 2014, 8, 2045–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Identification and concentration of some flavonoid components in Malaysian young ginger (Zingiber officinale Roscoe) varieties by a high performance liquid chromatography method. Molecules 2010, 15, 6231–6243. [Google Scholar] [CrossRef]
- Gong, F.; Fung, Y.S.; Liang, Y.Z. Determination of volatile components in ginger using gas chromatography-mass spectrometry with resolution improved by data processing techniques. J. Agric. Food Chem. 2004, 52, 6378–6383. [Google Scholar] [CrossRef]
- Shao, X.; Lv, L.; Parks, T.; Wu, H.; Ho, C.T.; Sang, S. Quantitative analysis of ginger components in commercial products using liquid chromatography with electrochemical array detection. J. Agric. Food Chem. 2010, 58, 12608–12614. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Snook, H.D.; Tareq, F.S.; Fasina, Y. Precision Research on Ginger: The Type of Ginger Matters. J. Agric. Food Chem. 2020, 68, 8517–8523. [Google Scholar] [CrossRef]
- Wu, H.; Tang, Z.; Qiu, L.; Ye, D. The comparison of component contents and pharmacological actions between two kinds of processed products of Pinellia rhizoma prepared by ginger juice and alum. Zhongguo Zhong Yao Za Zhi 1999, 24, 25–28, 63. [Google Scholar]
- Zhong, L.Y.; Su, D.; Zhu, J.; Deng, Y.F. Comparison of Coptidis Rhizoma processed with different ginger juice based on metabolomics. Zhongguo Zhong Yao Za Zhi 2016, 41, 2712–2719. [Google Scholar]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Marzec, Z.; Kasperek, E.; Wyszogrodzka-Koma, L.; Szwerc, W.; Asakawa, Y. Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations. Int. J. Mol. Sci. 2017, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Wang, Z.H.; He, F.; Meng, H.; Peng, J.H.; Shi, J.L. Analysis of Volatile Oils from Different Processed Products of Zingiber officinale Rhizome by GC-MS. Zhong Yao Cai 2015, 38, 723–726. [Google Scholar] [PubMed]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1011, 223–232. [Google Scholar] [CrossRef]
- Wu, C.Y.; Kong, M.; Zhang WLong, F.; Zhou, J.; Zhou, S.S.; Xu, J.D.; Xu, J.; Li, S.L. Impact of sulphur fumigation on the chemistry of ginger. Food Chem. 2018, 239, 953–963. [Google Scholar] [CrossRef]
- Li, M.Q.; Hu, X.Y.; Wang, Y.Z.; Zhang, X.J.; Li, J.P.; Song, Z.M.; Liu, Y.F.; Feng, W.S. Qualitative analysis on chemical constituents from different polarity extracted fractions of the pulp and peel of ginger rhizomes by ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e9029. [Google Scholar]
- Tao, Y.; Li, W.; Liang, W.; Van Breemen, R.B. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 10014–10021. [Google Scholar] [CrossRef]
- Jiang, H.; Solyom, A.M.; Timmermann, B.N.; Gang, D.R. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2957–2964. [Google Scholar] [CrossRef]
- Jiang, H.; Timmermann, B.N.; Gang, D.R. Characterization and identification of diarylheptanoids in ginger (Zingiber officinale Rosc.) using high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 509–518. [Google Scholar] [CrossRef]
- Li, T.; Pan, D.B.; Pang, Q.Q.; Zhou, M.; Yao, X.J.; Yao, X.S.; Li, H.B.; Yu, Y. Diarylheptanoid analogues from the rhizomes of Zingiber officinale and their anti-tumour activity. RSC Adv. 2021, 11, 29376–29384. [Google Scholar] [CrossRef]
- Riethmuller, E.; Alberti, A.; Toth, G.; Beni, S.; Ortolano, F.; Kery, A. Characterisation of diarylheptanoid- and flavonoid-type phenolics in Corylus avellana L. leaves and bark by HPLC/DAD-ESI/MS. Phytochem. Anal. 2013, 24, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Riethmuller, E.; Toth, G.; Alberti, A.; Vegh, K.; Burlini, I.; Konczol, A.; Balogh, G.T.; Kéry, Á. First characterisation of flavonoid- and diarylheptanoid-type antioxidant phenolics in Corylus maxima by HPLC-DAD-ESI-MS. J. Pharm. Biomed. Anal. 2015, 107, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of ginger extracts obtained by subcritical water. J. Supercrit. Fluids. 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Ni, J.; Xu, L.; Li, W.; Wu, L. Simultaneous determination of thirteen kinds of amino acid and eight kinds of acylcarnitine in human serum by LC-MS/MS and its application to measure the serum concentration of lung cancer patients. Biomed. Chromatogr. 2016, 30, 1796–1806. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Ho, C.T.; Huang, Q.; Song, S. Development of a solid phase microextraction protocol for the GC-MS determination of volatile off-flavour compounds from citral degradation in oil-in-water emulsions. Food Chem. 2013, 141, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Ruiz-Sala, P.; Vicente, Y.; Merinero, B.; Perez-Cerda, C.; Ugarte, M. Separation and identification of plasma short-chain acylcarnitine isomers by HPLC/MS/MS for the differential diagnosis of fatty acid oxidation defects and organic acidemias. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 860, 121–126. [Google Scholar] [CrossRef]
- Han, J.S.; Lee, S.; Kim, H.Y.; Lee, C.H. MS-Based Metabolite Profiling of Aboveground and Root Components of Zingiber mioga and Officinale. Molecules 2015, 20, 16170–16185. [Google Scholar] [CrossRef]
- Fang, W.T.; Zhan, Z.L.; Peng, H.S.; Huang, L.Q. Historical evolution and change of differentiation on dried ginger, fresh ginger and baked ginger. Zhongguo Zhong Yao Za Zhi 2017, 42, 1641–1645. [Google Scholar]
- Wang, L.; Fang, L.; Zhao, H.; Wang, S.; Du, J.; Wang, X. Analysis of gingerol-related compounds in fresh ginger by HPLC-ESI-Q-TOF-MS/MS. Zhongguo Zhong Yao Za Zhi 2011, 36, 3467–3471. [Google Scholar]






| No. | Compound Name | Formula | Rt/ min | Detected Mass | Expected Tgt Mass | Diff/ ppm | Positive | Negative |
|---|---|---|---|---|---|---|---|---|
| MS/MS | MS/MS | |||||||
| 1 | Isoleucine | C6H13NO2 | 0.50 | 131.0947 | 131.0946 | 0.14 | N | 130.0870[M–H]−, 115.0035[M–NH2]−, 71.0140[M–NH2–COO]− |
| 2 | Phenylalanine | C9H11NO2 | 0.69 | 165.0785 | 165.0790 | −2.70 | N | 164.0713[M–H]−, 147.8934[M–H2O]−, 103.0557[M–H2O–COO]−, and 72.0098 |
| 3 | (Z)-citral | C10H16O | 2.66 | 152.1197 | 152.1201 | −2.88 | 153.1282[M+H]+, 93.0332[M+H-CH2COO]+, and 65.0383[M+H–CH2COO–CO]+ | N |
| 4 | Galanganol C | C27H28O5 | 4.07 | 432.1937 | 432.1937 | 12.83 | N | 431.1914[M–H]−, 389.1797, 179.0545, 89.0243 |
| 5 | Zingerone * | C11H14O3 | 6.71 | 194.0942 | 194.0943 | 1.86 | N | 193.0863[M–H]−, 178.0573 [M–CH3–H]−, and 135.0450 |
| 6 | Dihydrocurcumin | C21H22O6 | 7.75 | 370.1402 | 370.1416 | −3.80 | 371.1481[M+H]+, 235.0943, 177.0535, 137.0589 | 369.1251[M–H]− |
| 7 | Tetrahydrocurcumin | C21H24O6 | 11.58 | 372.1557 | 372.1573 | −4.18 | 373.1578[M+H]+, 179.0963, 153.0537, and 137.0589 | |
| 8 | (E)-7-(3,4-dihydroxyphenyl)-1-(4-hydroxy-3-methoxyphenyl)hept-2-en-1-one | C20H22O5 | 12.00 | 342.1451 | 342.1467 | −4.64 | 343.1525[M+H]+, 258.2470, 147.0438, 137.0586, 123.0431, 107.0481, and 86.0960 | 327.1564[M–H]−, 135.0441 |
| 9 | 1,5-epoxy-3-hydroxy-1-(3,4-dihydroxy-5-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane | C21H26O7 | 12.18 | 390.1659 | 390.1679 | −5.09 | 391.1731[M+H]+, 179.0689, and 137.0586 | 389.1616[M–H]−, 165.0554 |
| 10 | 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-3-heptanone | C20H24O5 | 12.23 | 344.1599 | 344.1624 | −7.13 | 345.1631[M+H]+, 258.1470, and 123.0431 | 343.1552[M–H]− |
| 11 | Methyl diacetoxy-[4]-gingerdiol | C20H30O6 | 13.73 | 366.2048 | 366.2042 | 1.39 | 389.1952[M+Na]+, 355.1524, 297.1104, 193.0484, and 137.0588 | N |
| 12 | 3,5-dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane | C22H30O7 | 13.73 | 406.1973 | 406.1992 | −4.50 | 407.1992[M+H]+, 215.1382, 137.0584, 86.0959, and 70.0650 | 405.1530[M–H]−, 165.0558 |
| 13 | [4]-Shogaol | C15H20O3 | 14.00 | 248.1403 | 248.1412 | −3.84 | 249.1481[M+H]+, 163.0745, 137.0590, and 131.0480 | N |
| 14 | 1,5-epoxy-3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-4-methoxyphenyl)heptane | C22H28O7 | 14.86 | 404.1819 | 404.1835 | −3.90 | 405.1885[M+H]+, 217.1210, 167.0693, and 139.0854 | N |
| 15 | Gingerenone B | C22H26O6 | 14.87 | 386.1714 | 386.1729 | −4.01 | 387.1729[M+H]+, 247.1316, 193.0848, 167.0692, and 137.0592 | 385.1603[M–H]−, 341.1071, 223.0609, and 101.0248, |
| 16 | 3-acetoxy-5-hydroxy-1-(4-hydroxyphenyl)-7-(3,4-dihydroxyphenyl)heptane | C21H26O6 | 15.04 | 374.1709 | 374.1729 | −5.43 | 375.1780[M+H]+, 341.1720, 217.1207, 163.0744, and 137.0588 | 373.1661[M–H]−, 331.1540, and 175.0753 |
| 17 | Dihydro-[6]-paradol | C17H28O3 | 15.64 | 280.2050 | 280.2038 | 4.13 | 303.1941[M+Na]+, 287.1989, 163.0742, and 103.0383 | N |
| 18 | 3,5-diacetoxy-1,7-bis(3,4-dihydroxy-5-methoxyphenyl)heptane | C25H32O10 | 15.96 | 492.1971 | 492.1996 | −5.00 | 510.2883, 235.1176, 137.0589, 110.0708 | N |
| 19 | Isomer of number 18 | C25H32O10 | 16.10 | 492.1973 | 492.1996 | −4.41 | 510.2883, 235.1176, 137.0589, and 110.0708 | N |
| 20 | 3-acetoxy-5-hydroxy-1-(3,4-dihydroxy-5-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane | C23H30O8 | 17.01 | 434.1924 | 434.1941 | −3.89 | 435.1989[M+H]+, 385.1987, 357.1692, 207.1003, 193.0846,181.0847,167.0694,163.0745,153.0539, and 137.0590 | N |
| 21 | 3,5-diacetoxy-1-(3,4-dihydroxyphenyl)-7(4-hydroxyphenyl)heptane | C23H28O7 | 17.01 | 416.1817 | 416.1835 | −4.37 | 417.1890[M+H]+, 324.1414, 217.1211, 207.1009, 153.0539, 137.0590, and 81.0694 | 415.1761[M-H]−, 371.2049, and 359.1597 |
| 22 | Isomer of number 45 | C22H34O6 | 17.45 | 394.2334 | 394.2355 | N | N | N |
| 23 | 3-acetoxy-5-hydroxy-1-(3,4-dihydroxy-5-methoxyphenyl)heptane | C24H32O8 | 17.97 | 448.2075 | 448.2097 | −4.93 | 449.2141[M+H]+, 373.1634, 313.1423,123.0435, | N |
| 24 | 3,5-diacetoxy-1-(3,4-dihydroxyphenyl)-7-(3,4-dihydroxy-5-methoxyphenyl)heptane | C24H30O9 | 18.06 | 462.1860 | 462.1890 | −6.41 | 480.2202, 179.0685, and 137.0593 | N |
| 25 | Dehydro-[6]-gingerdione | C17H22O4 | 18.07 | 290.1526 | 290.1518 | 2.86 | 177.0538, 145.0277 | N |
| 26 | Isomer of number 18 | C25H32O10 | 18.38 | 492.1969 | 492.1996 | −4.38 | 510.2883, 235.1176, 137.0589, and 110.0708 | N |
| 27 | unknown | unknown | 18.71 | 466.2413 | 466.2413 | 2.34 | Unknown | N |
| 28 | Trihydroxy octadecenoic acid | C18H34O5 | 18.72 | 330.2405 | 330.2406 | −0.40 | N | 329.233[M-H]−, 283.2613 |
| 29 | Curcumadiol | C15H26O2 | 19.24 | 238.1946 | 238.1933 | 7.94 | 261.1839[M+Na]+, 229.1566, 177.0886, 163.0743, 145.0637, 137.0590, 131.0488, 117.0692, and 103.0536 | N |
| 30 | 3,5-diacetoxy-1-(3,4-dihydroxy-5-methoxyphenyl)-7-(4-hydroxy-3,5-dimethoxyphenyl)heptane | C26H34O10 | 19.64 | 506.2129 | 506.2152 | −4.61 | 507.2168[M+H]+, 355.1523, 215.1054, 179.0695, and 137.0587 | N |
| 31 | [6]-Paradol | C17H26O3 | 19.91 | 278.1869 | 278.1882 | −4.74 | 279.0941[M+H]+, 261.1840[M+H–H2O]+, 233.0954[M+H–H2O–CO]+, and 137.0595 | N |
| 32 | 3,5-diacetoxy-1-(4-hydroxy-3-methoxyphenyl)-7-(3, 4-dihydroxy-5-methoxyphenyl)heptane | C25H32O9 | 19.93 | 476.2032 | 476.2046 | −2.94 | 477.2855[M+H]+, 285.2163, 179.0695, 137.0591, and 69.0693 | N |
| 33 | [6]-Gingerol* | C17H26O4 | 20.24 | 294.1819 | 294.1831 | −4.10 | 317.1712[M+Na]+, 177.0906, 137.0600, 99.0797, 69.0697 | N |
| 34 | 6-hydroxy-[6]-shogaol | C17H24O4 | 20.63 | 292.1664 | 292.1675 | −3.79 | 293.1742[M+H]+, 179.0690, and 137.0588 | N |
| 35 | 3,5-diacetoxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane | C26H34O9 | 21.60 | 490.2176 | 490.2203 | −5.53 | 491.2525[M+H]+, 431.2055[M+H–CH3COO]+, 371.1837[M+H–2CH3COO]+, 339.1577, 247.1314, and 193.0852 | N |
| 36 | 3,5-diacetoxy-1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)heptane | C24H30O7 | 21.74 | 430.1969 | 430.1992 | −5.31 | 431.2926[M+H]+, 193.0848, and 167.0691 | N |
| 37 | 1,7bis-(4-hydroxy-3-methoxyphenyl)-5-methoxy-3-heptanone | C22H28O6 | 21.83 | 388.1887 | 388.1886 | 0.15 | N | 387.1814[M–H]−, 329.1384, 207.1025, 165.0552, and 122.0372 |
| 38 | 3,5-diacetoxy-1,7-bis(4-hydroxy-3-methoxyphenyl)heptane | C25H32O8 | 22.01 | 460.2078 | 460.2097 | −4.10 | 478.2418[M+NH4]+, 341.1732, 217.1212, and 137.0593 | N |
| 39 | Palmitic acid | C16H32O2 | 22.16 | 256.2392 | 256.2402 | −4.03 | 257.2620[M+H]+, 191.1054, and 106.0856 | N |
| 40 | Diacetoxy-[4]-gingerdiol | C19H28O6 | 23.35 | 352.1863 | 352.1886 | −6.48 | 370.2203[M+H2O]+, 137.0591 | N |
| 41 | Acetoxy-[6]-gingerol | C19H28O5 | 25.12 | 336.1921 | 336.1937 | −4.82 | 337.1985[M+H]+, 279.0995, 261.0898, 163.0739, 137.0590, 131.0484, 122.0359, 103.0537, and 94.0409 | N |
| 42 | [6]-shogaol* | C17H24O3 | 25.24 | 276.1719 | 276.1725 | −4.19 | 277.1787[M+H]+, 137.0591, 122.0356, and 94.0408 | N |
| 43 | Diacetoxy-[6]-gingerdiol | C21H32O6 | 27.14 | 380.2178 | 380.2199 | −5.60 | 403.2071[M+Na]+, 321.2052 [M+H-CH3COO]+, 137.0592 | N |
| 44 | Dehydro-[8]-gingerol | C19H28O4 | 27.14 | 320.1975 | 320.1988 | −3.93 | 321.2049[M+H]+, 261.1840, 177.0904,163.0742, and 137.0590 | N |
| 45 | Methyl,diacetoxy-[6]-gingerdiol | C22H34O6 | 28.96 | 394.2334 | 394.2355 | −5.39 | 417.2227[M+Na]+, 412.2678[M+H2O]+, 335.2210[M+H–CH3COO]+, 275.1911[M+H–2CH3COO]+, 177.0900, 151.0747, 137.0588, | N |
| 46 | Isomer of number 21 | C23H28O7 | 29.40 | 416.1817 | 416.1835 | N | 417.1890[M+H]+, 324.1414, 217.1211, 207.1009, 153.0539, and 137.0590 | N |
| 47 | Dehydro-10-gingerdione | C21H30O4 | 29.55 | 346.2141 | 346.2144 | −0.82 | 347.2206[M+H]+, 177.0902, and 137.0592 | N |
| 48 | [6]-gingerdiol | C17H28O4 | 31.20 | 296.1997 | 296.1988 | 3.27 | 319.1890[M+Na]+, 177.0899, 137.0589, and 94.0399 | N |
| 49 | [10]-shogaol | C21H32O3 | 32.11 | 332.2334 | 332.2351 | −5.14 | 333.2410[M+H]+, 137.0590, 94.0403 | N |
| 50 | Acetoxy-[10]-gingerol | C23H36O5 | 32.11 | 392.2537 | 392.2563 | −6.59 | 393.2606[M+H]+, 195.1220, 163.0737, and 137.0592 | N |
| 51 | 6-hydroxy-[10]-shogaol | C21H32O4 | 34.06 | 348.2284 | 348.2301 | −4.88 | 349.2357[M+H]+, 179.0696, 161.0949, 137.0588, 121.0587, 95.0847 | 347.2227[M–H]− |
| 52 | Oleamide | C18H35NO | 34.16 | 281.2708 | 281.2719 | −3.82 | 282.2781[M+H]+, and 187.0727 | N |
| 53 | Dehydro-[12]-gingerdione | C23H34O4 | 37.81 | 374.2440 | 374.2457 | −4.52 | 375.2514[M+H]+, 177.0901, and 137.0590 | 373.1646[M–H]−, 313.1437, 191.1078, and 122.0374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Cai, H.; Li, S.; Xie, W.; Sun, R. The Chemical Signatures of Water Extract of Zingiber officinale Rosc. Molecules 2022, 27, 7818. https://doi.org/10.3390/molecules27227818
Lu F, Cai H, Li S, Xie W, Sun R. The Chemical Signatures of Water Extract of Zingiber officinale Rosc. Molecules. 2022; 27(22):7818. https://doi.org/10.3390/molecules27227818
Chicago/Turabian StyleLu, Fengying, Hua Cai, Saimei Li, Wei Xie, and Rongjin Sun. 2022. "The Chemical Signatures of Water Extract of Zingiber officinale Rosc" Molecules 27, no. 22: 7818. https://doi.org/10.3390/molecules27227818
APA StyleLu, F., Cai, H., Li, S., Xie, W., & Sun, R. (2022). The Chemical Signatures of Water Extract of Zingiber officinale Rosc. Molecules, 27(22), 7818. https://doi.org/10.3390/molecules27227818