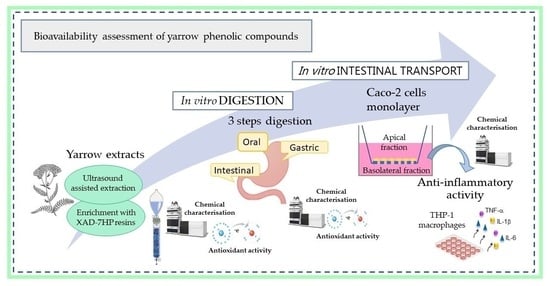
Bioavailability Assessment of Yarrow Phenolic Compounds Using an In Vitro Digestion/Caco-2 Cell Model: Anti-Inflammatory Activity of Basolateral Fraction
Abstract
:1. Introduction
2. Results and Discussions
2.1. Influence of In Vitro Gastrointestinal Steps on Phenolic Composition and Antioxidant Activity of the Extracts
2.2. Caco-2 Cells Transport Experiments
2.3. Anti-Inflammatory Activity of Caco-2 Cells Basolateral Fraction
3. Materials and Methods
3.1. Yarrow Extract and Yarrow Phenolic Compounds-Enriched Extract Obtention
3.2. HPLC-PAD Phenolic Compounds Analysis
3.3. Determination of Total Phenolic Content (TPC) and Antioxidant Activity
3.4. In Vitro Gastrointestinal Digestion
3.5. Caco-2 Cells Culture and Transport Experiments
3.6. Anti-Inflammatory Assays of Basolateral Fraction from Caco-2 Experiments
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barda, C.; Grafakou, M.E.; Tomou, E.M.; Skaltsa, H. Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021). Sci. Pharm. 2021, 89, 50. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical composition of essential oils and extracts of Achillea species and their biological activities: A review. J. Etnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Benetis, R.; Bumblauskie, L.; Burdulis, D.; Janulis, V.; Toleikis, A.; Viskelis, P.; Jakstas, V. Achillea millefolium L. sl herb extract: Antioxidant activity and effect on the rat heart mitochondrial functions. Food Chem. 2011, 127, 1540–1548. [Google Scholar] [CrossRef]
- Pereira, J.M.; Peixoto, V.; Teixeira, A.; Sousa, D.; Barros, L.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cells lines by interfering with cell cycle and inducing apoptosis. Food Chem. Toxicol. 2018, 118, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.F.; Muhammad, J.S.; Shahryar, S.; Usmanghani, K.; Gilani, A.H.; Jafri, W.; Sugiyama, T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012, 141, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Abdossi, V.; Kazemi, M. Bioactivities of Achillea millefolium essential oil and its main terpenes from Iran. Int. J. Food Prop. 2016, 19, 1798–1808. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.I.; Morales, P.; Barreira, J.C.; Oliveira, M.B.P.; Sánchez-Mata, M.C.; Ferreira, I.C. Minerals and vitamin B9 in dried plants vs. infusions: Assessing absorption dynamics of minerals by membrane dialysis tandem in vitro digestion. Food Biosci. 2016, 13, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Goulas, V.; Hadjisolomou, A. Dynamic changes in targeted phenolic compounds and antioxidant potency of carob fruit (Ceratonia siliqua L.) products during in vitro digestion. LWT 2019, 101, 269–275. [Google Scholar] [CrossRef]
- Lima, K.; Silva, O.; Figueira, M.E.; Pires, C.; Cruz, D.; Gomes, S.; Mauricio, E.M.; Duarte, M.P. Influence of the in vitro gastrointestinal digestion on the antioxidant activity of Artemisia gorgorum Webb and Hyptis pectinata (L.) Poit. Infusions from Cape Verde. Food Res. Int. 2019, 115, 150–159. [Google Scholar] [CrossRef]
- Arranz, E.; Corredig, M.; Guri, A. Designing food delivery systems: Challenges related to the in vitro methods employed to determine the fate of bioactives in the gut. Food Funct. 2016, 7, 3319–3336. [Google Scholar] [CrossRef]
- Lingua, M.S.; Theumer, M.G.; Kruzynski, P.; Wunderlin, D.A.; Baroni, M.V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: Comparative study with its winemaking product. Food Res. Int. 2019, 122, 496–505. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Ruíz-Riaguas, A.; Fernández-de Córdova, M.L.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phenolic profile and antioxidant activity of Jasonia glutinosa herbal tea. Influence of simulated gastrointestinal in vitro digestion. Food Chem. 2019, 287, 258–264. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castillo, P.C. Antioxidant polyphenols of Madeira sorrel (Rumex maderensis): How do they survive to in vitro simulated gastrointestinal digestion? Food Chem. 2018, 259, 105–112. [Google Scholar] [CrossRef]
- Bowles, S.L.; Ntamo, Y.; Malherbe, C.J.; Kappo, A.M.; Louw, J.; Muller, C.J. Intestinal transport and absorption of bioactive phenolic compounds from a chemically characterized aqueous extract of Athrixia phylicoides. J. Ethnopharmacol. 2017, 200, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Grootaert, C.; Voorspoels, S.; Jacobs, G.; Pitart, J.; Kamiloglu, S.; Possemiens, S.; Heinonen, M.; Kardum, N.; Glibetic, M.; et al. Aronia (Aronia melanocarpa) phenolics bioavailability in a combined in vitro digestion/Caco-2 cell model is structure and colon region dependent. J. Funct. Foods 2017, 38, 128–139. [Google Scholar] [CrossRef]
- Xu, C.; Kong, L.; Tian, Y. Investigation of the Phenolic Component Bioavailability Using the In Vitro Digestion/Caco-2 Cell Model, as well as the Antioxidant Activity in Chinese Red Wine. Foods 2022, 11, 3108. [Google Scholar] [CrossRef]
- Goltz, C.; Avila, S.; Barbieri, J.B.; Igarashi-Mafra, L.; Mafra, M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satueioides) extracts. Ind. Crops Prod. 2018, 115, 227–234. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Larrán, P.; Díaz-Reinoso, B.; Moure, A.; Alonso, J.L.; Domínguez, H. Adsorption technologies to recover and concentrate food polyphenols. Curr. Opin. Food Sci. 2018, 23, 165–172. [Google Scholar] [CrossRef]
- Villalva, M.; Jaime, L.; Aguado, E.; Nieto, J.A.; Reglero, G.; Santoyo, S. Anti-inflammatory and antioxidant activities from the basolateral fraction of Caco-2 cells exposed to a rosmarinic acid enriched extract. J. Agric. Food Chem. 2018, 66, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Santoyo, S.; Salas-Perez, L.; Siles-Sanchez, M.N.; García-Risco, M.R.; Formari, T.; Reglero, G.; Jaime, L. Sustainable extraction techniques for obtaining antioxidant and anti-inflammatory compounds from the Lamiaceae and Asteraceae species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef]
- Bouayed, J.; DeuBer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialyzable polyphenols in selected apple varieties following in vitro digestion vs. native patters. Food Chem. 2012, 131, 1466–1742. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Xia, Y.; Li, H.; Shi, X.; Wang, J.; Deng, Z. Bioaccessibility and transformation pathways of phenolics compounds in processed mulberry (Morus alba L.) leaves after in vitro gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2019, 60, 103406. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; León-Félix, J.; Heredia, B. Effect of in vitro digestion on the total antioxidant capacity and phenolic content of 3 species of oregano (Hedeoma patents, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Song, D.-G.; Pan, C.-H.; Lee, W.J.; Um, B.H. Isolation and identification of antioxidant compounds from Ligularia fischeri. J. Food Sci. 2010, 75, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, M.; Pan, H.; Sun, S.; Li, L.; Zeng, S.; Jiang, H. Role of catechol-O-methyltransferase in the disposition of luteolin in rats. Drug Metab. Dispos. 2011, 39, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Čvorović, J.; Ziberna, L.; Fornasaro, S.; Tramer, F.; Passamonti, S. Bioavailability of Flavonoids: The Role of Cell Membrane Transporters. In Polyphenols: Mechanisms of Action in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 295–320. [Google Scholar]
- Zhou, W.; Shan, J.; Wang, S.; Cai, B.; Di, L. Transepithelial transport of phenolic acids in Flos lonicerae japonicae in intestinal Caco-2 cell monolayers. Food Funct. 2015, 6, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. Biofactors 2013, 39, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef]
- Garg, M.; Chaudhary, S.K.; Goyal, A.; Sarup, P.; Kumari, S.; Garg, N.; Vaid, L.; Shiveena, B. Comprehensive review on therapeutic and phytochemical exploration of diosmetin: A promising moiety. Phytomed. Plus 2022, 2, 100179. [Google Scholar] [CrossRef]
- Gubbiveeranna, V.; Nagaraju, S. Ethnomedicinal, phytochemical constituents and pharmacological activities of Tridax procumbens: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 1–7. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Reventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Willians, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Uchida, M.; Fukazawa, T.; Yamazaki, Y.; Hashimoto, H.; Miyamoto, Y. A modified fast (4 day) 96-well plate Caco-2 permeability assay. J. Pharmacol. Toxicol. Methods 2009, 59, 39–43. [Google Scholar] [CrossRef]


| Compound | Undigested YE | Oral | Gastric | Intestinal |
|---|---|---|---|---|
| Neochlorogenic acid | 0.24 ± 0.11 b | 0.21 ± 0.06 b | 0.29 ± 0.09 b | 0.56 ± 0.07 a |
| Protocatechuic acid | 0.13 ± 0.10 b | 0.12 ± 0.07 b | 0.13 ± 0.08 b | 0.47 ± 0.12 a |
| Caftaric acid isomer | 0.08 ± 0.03 a | 0.08 ± 0.04 a | 0.06 ± 0.03 ab | 0.04 ± 0.03 b |
| Caftaric acid | 0.07 ± 0.03 a | 0.07 ± 0.04 a | 0.18 ± 0.09 a | 0.13 ± 0.07 |
| Caffeoylquinic acid isomer I | 0.39 ± 0.09 a | 0.39 ± 0.08 a | 0.24 ± 0.08 ab | 0.22 ± 0.06 b |
| Chlorogenic acid | 5.67 ± 0.25 a | 5.02 ± 0.21 ab | 5.90 ± 0.30 a | 4.75 ± 0.20 b |
| Cryptochlorogenic acid | 0.13 ± 0.05 b | 0.10 ± 0.03 b | 0.17 ± 0.04 b | 0.75 ± 0.12 a |
| Vicenin 2 | 2.11 ± 0.10 bc | 2.02 ± 0.10 c | 2.45 ± 0.15 a | 2.24 ± 0.10 ab |
| Caffeoylquinic acid isomer II | 0.10 ± 0.03 a | 0.12 ± 0.04 a | 0.10 ± 0.02 a | 0.10 ± 0.03 a |
| Apigenin hexoside-pentoside I | 0.46 ± 0.06 a | 0.48 ± 0.05 a | 0.49 ± 0.06 a | 0.43 ± 0.06 a |
| Caffeic acid | 0.34 ± 0.04 a | 0.36 ± 0.06 a | 0.40 ± 0.05 a | 0.42 ± 0.06 a |
| Schaftoside isomer | 1.34 ± 0.10 a | 1.32 ± 0.09 a | 1.43 ± 0.10 a | 1.43 ± 0.12 a |
| Schaftoside | 1.77 ± 0.18 ab | 1.61 ± 0.15 b | 2.14 ± 0.19 a | 2.01 ± 0.16 a |
| Homoorientin | 2.10 ± 0.19 a | 1.94 ± 0.12 a | 2.20 ± 0.15 a | 1.89 ± 0.12 a |
| Apigenin hexoside-pentoside II | 1.04 ± 0.11 a | 0.97 ± 0.09 a | 1.04 ± 0.10 a | 0.98 ± 0.08 a |
| Luteolin dihexoside I | 2.60 ± 0.18 ab | 2.32 ± 0.12 b | 2.77 ± 0.11 a | 2.52 ± 0.11 b |
| 6-hydroxyluteolin-7-O-glucoside | 2.03 ± 0.12 b | 1.97 ± 0.08 b | 2.34 ± 0.12 a | 1.74 ± 0.09 c |
| Apigenin dihexoside | 0.15 ± 0.09 a | 0.16 ± 0.06 a | 0.21 ± 0.07 a | 0.16 ± 0.04 a |
| Quercetin hexoside | 1.33 ± 0.13 a | 1.31 ± 0.08 a | 1.10 ± 0.10 a | 0.25 ± 0.07 b |
| Luteolin dihexoside II | 0.23 ± 0.04 a | 0.23 ± 0.06 a | 0.27 ± 0.07 a | 0.24 ± 0.04 a |
| Rutin | 1.06 ± 0.07 a | 1.08 ± 0.09 a | 1.16 ± 0.07 a | 1.02 ± 0.09 a |
| Apigenin hexoside | 0.50 ± 0.04 a | 0.47 ± 0.07 a | 0.57 ± 0.06 a | 0.52 ± 0.06 a |
| Vitexin | 0.67 ± 0.07 a | 0.61 ± 0.09 a | 0.72 ± 0.08 a | 0.64 ± 0.07 a |
| Apigenin hexoside-deoxyhexoside | 0.40 ± 0.05 a | 0.42 ± 0.04 a | 0.25 ± 0.04 b | 0.22 ± 0.03 b |
| Apigenin derivative | 2.52 ± 0.12 b | 2.49 ± 0.09 b | 2.54 ± 0.10 b | 2.72 ± 0.11 a |
| Luteolin-7-O-glucoside | 8.29 ± 0.28 a | 8.12 ± 0.32 a | 6.70 ± 0.25 c | 7.24 ± 0.33 b |
| Luteolin-7-O-glucuronide | 0.72 ± 0.09 a | 0.69 ± 0.08 ab | 0.57 ± 0.05 b | 0.69 ± 0.07 ab |
| Quercetin hexuronide | 0.15 ± 0.03 b | 0.12 ± 0.05 b | 0.25 ± 0.03 a | 0.20 ± 0.04 ab |
| 3,4-Dicaffeoylquinic acid | 1.49 ± 0.10 b | 1.37 ± 0.08 b | 1.42 ± 0.08 b | 6.26 ± 0.27 a |
| Isorhamnetin hexoside I | 1.59 ± 0.12 a | 1.49 ± 0.09 a | 1.00 ± 0.07 b | 1.00 ± 0.06 b |
| 1,5-Dicaffeoylquinic acid | 1.65 ± 0.11 a | 1.66 ± 0.10 a | 1.49 ± 0.07 ab | 1.37 ± 0.08 b |
| 3,5-Dicaffeoylquinic acid | 23.8 ± 1.81 a | 22.9 ± 1.13 a | 18.8 ± 0.90 b | 8.77 ± 0.11 c |
| Apigenin-7-O-glucoside | 2.27 ± 0.10 a | 2.15 ± 0.07 ab | 2.01 ± 0.08 b | 1.81 ± 0.09 c |
| Luteolin-O-malonylglucoside | 0.53 ± 0.04 a | 0.52 ± 0.03 a | 0.50 ± 0.04 ab | 0.44 ± 0.03 b |
| 4,5-Dicaffeoylquinic acid | 4.25 ± 0.20 b | 4.05 ± 0.18 b | 3.61 ± 0.12 c | 11.5 ± 0.51 a |
| Isorhamnetin hexoside II | 0.62 ± 0.06 b | 0.60 ± 0.04 b | 0.50 ± 0.04 c | 1.35 ± 0.10 a |
| Dicaffeoylquinic acid isomer | 0.06 ± 0.01 b | 0.05 ± 0.02 b | 0.06 ± 0.02 b | 0.10 ± 0.01 a |
| Feruloylcaffeoylquinic acid | 0.14 ± 0.03 a | 0.12 ± 0.02 a | 0.07 ± 0.02 b | 0.11 ± 0.03 ab |
| Tricaffeoylquinic acid | 0.36 ± 0.06 a | 0.31 ± 0.04 ab | 0.09 ± 0.01 c | 0.25 ± 0.04 b |
| Luteolin | 1.90 ± 0.10 a | 1.94 ± 0.11 a | 0.95 ± 0.08 c | 1.32 ± 0.10 b |
| Quercetin | 0.63 ± 0.05 a | 0.60 ± 0.07 a | 0.29 ± 0.06 b | 0.16 ± 0.04 c |
| Methoxyquercetin | 0.36 ± 0.03 a | 0.34 ± 0.04 ab | 0.26 ± 0.04 b | 0.32 ± 0.04 ab |
| Apigenin | 0.56 ± 0.05 a | 0.58 ± 0.04 a | 0.18 ± 0.02 c | 0.38 ± 0.05 b |
| Diosmetin | 0.40 ± 0.05 a | 0.38 ± 0.04 a | 0.22 ± 0.03 c | 0.29 ± 0.04 b |
| Trihydroxy dimethoxyflavone | 0.27 ± 0.02 a | 0.29 ± 0.02 a | 0.13 ± 0.01 c | 0.20 ± 0.02 b |
| Centaureidin | 2.02 ± 0.12 a | 2.07 ± 0.09 a | 1.22 ± 0.05 c | 1.76 ± 0.08 b |
| Methoxyacacetin | 0.25 ± 0.03 a | 0.26 ± 0.02 a | 0.09 ± 0.02 c | 0.16 ± 0.02 b |
| Dihydroxy trimethoxyflavone | 0.44 ± 0.05 a | 0.46 ± 0.06 a | 0.17 ± 0.03 c | 0.31 ± 0.05 b |
| Casticin | 2.93 ± 0.10 a | 2.92 ± 0.11 a | 1.45 ± 0.09 c | 2.31 ± 0.10 b |
| Compound | Undigested-EE | Oral | Gastric | Intestinal |
|---|---|---|---|---|
| Neochlorogenic acid | 0.15 ± 0.03 c | 0.15 ± 0.02 c | 0.22 ± 0.04 b | 0.86 ± 0.06 a |
| Protocatechuic acid | 0.13 ± 0.02 b | 0.13 ± 0.03 b | 0.14 ± 0.03 b | 0.74 ± 0.07 a |
| Caftaric acid isomer | 0.15 ± 0.02 a | 0.13 ± 0.02 a | 0.05 ± 0.01 b | 0.05 ± 0.02 b |
| Caftaric acid | 0.19 ± 0.06 b | 0.18 ± 0.05 b | 0.30 ± 0.08 a | 0.30 ± 0.06 a |
| Caffeoylquinic acid isomer I | 0.46 ± 0.07 a | 0.42 ± 0.06 a | 0.48 ± 0.07 a | 0.39 ± 0.06 a |
| Chlorogenic acid | 7.60 ± 0.35 a | 7.46 ± 0.21 a | 7.49 ± 0.01 a | 6.28 ± 0.25 b |
| Cryptochlorogenic acid | 0.12 ± 0.01 b | 0.14 ± 0.02 b | 0.15 ± 0.02 b | 1.11 ± 0.07 a |
| Vicenin 2 | 3.20 ± 0.12 b | 3.22 ± 0.10 b | 3.37 ± 0.10 a | 3.49 ± 0.11 a |
| Caffeoylquinic acid isomer II | 0.22 ± 0.02 a | 0.24 ± 0.02 a | 0.27 ± 0.03 a | 0.28 ± 0.02 a |
| Apigenin hexoside-pentoside I | 0.76 ± 0.04 b | 0.77 ± 0.05 b | 0.96 ± 0.08 a | 0.80 ± 0.07 ab |
| Caffeic acid | 0.90 ± 0.06 a | 0.91 ± 0.06 a | 0.90 ± 0.04 a | 0.94 ± 0.05 a |
| Schaftoside isomer | 2.33 ± 0.14 b | 2.29 ± 0.11 b | 2.62 ± 0.12 a | 2.77 ± 0.10 a |
| Schaftoside | 3.64 ± 0.10 b | 3.57 ± 0.11 b | 4.02 ± 0.15 a | 3.92 ± 0.12 a |
| Homoorientin | 6.31 ± 0.21 a | 6.03 ± 0.16 a | 6.36 ± 0.18 a | 5.50 ± 0.13 b |
| Apigenin hexoside-pentoside II | 1.90 ± 0.10 a | 1.76 ± 0.09 a | 1.88 ± 0.08 a | 1.87 ± 0.09 a |
| Luteolin dihexoside I | 7.68 ± 0.19 a | 7.35 ± 0.12 b | 7.20 ± 0.10 b | 7.56 ± 0.11 ab |
| 6-hydroxyluteolin-7-O-glucoside | 6.46 ± 0.20 a | 6.25 ± 0.16 a | 6.58 ± 0.21 a | 5.26 ± 0.18 b |
| Apigenin dihexoside | 0.44 ± 0.08 | 0.41 ± 0.06 | 0.52 ± 0.06 | 0.42 ± 0.04 |
| Quercetin hexoside | 4.10 ± 0.20 a | 4.00 ± 0.14 a | 3.37 ± 0.15 b | 0.96 ± 0.10 c |
| Luteolin dihexoside II | 0.63 ± 0.05 b | 0.66 ± 0.04 b | 0.78 ± 0.06 a | 0.69 ± 0.04 ab |
| Rutin | 2.86 ± 0.11 b | 3.19 ± 0.12 a | 3.30 ± 0.10 a | 3.00 ± 0.13 ab |
| Apigenin hexoside | 1.75 ± 0.08 b | 2.06 ± 0.10 a | 2.25 ± 0.11 a | 2.24 ± 0.10 a |
| Vitexin | 2.51 ± 0.10 a | 2.44 ± 0.09 a | 2.52 ± 0.10 a | 2.42 ± 0.08 a |
| Apigenin hexoside- deoxyhexoside | 0.85 ± 0.04 a | 0.89 ± 0.05 a | 0.72 ± 0.04 b | 0.56 ± 0.03 c |
| Apigenin derivative | 6.44 ± 0.21 c | 6.80 ± 0.22 c | 7.60 ± 0.21 b | 8.23 ± 0.30 a |
| Luteolin-7-O-glucoside | 24.2 ± 1.30 a | 23.6 ± 1.12 a | 19.5 ± 1.06 c | 21.8 ± 1.02 b |
| Luteolin-7-O-glucuronide | 1.57 ± 0.08 a | 1.45 ± 0.07 a | 1.06 ± 0.06 c | 1.17 ± 0.09 b |
| Quercetin hexuronide | 0.95 ± 0.06 a | 0.88 ± 0.05 a | 0.87 ± 0.04 a | 0.97 ± 0.02 a |
| 3,4-Dicaffeoylquinic acid | 3.78 ± 0.18 b | 3.73 ± 0.12 b | 3.80 ± 0.10 b | 20.9 ± 1.22 a |
| Isorhamnetin hexoside I | 3.36 ± 0.10 a | 3.39 ± 0.09 a | 3.57 ± 0.11 a | 3.50 ± 0.12 a |
| 1,5-Dicaffeoylquinic acid | 4.29 ± 0.27 ab | 4.75 ± 0.21 a | 4.10 ± 0.12 b | 3.62 ± 0.14 c |
| 3,5-Dicaffeoylquinic acid | 72.4 ± 2.92 a | 72.5 ± 1.91 a | 60.4 ± 2.10 b | 24.2 ± 1.33 c |
| Apigenin-7-O-glucoside | 7.30 ± 0.33 a | 7.11 ± 0.21 a | 7.00 ± 0.18 a | 6.28 ± 0.15 b |
| Luteolin-O-malonylglucoside | 1.08 ± 0.08 a | 1.05 ± 0.05 a | 1.08 ± 0.07 a | 1.09 ± 0.08 a |
| 4,5-Dicaffeoylquinic acid | 13.3 ± 0.87 b | 12.6 ± 0.63 b | 10.5 ± 0.51 c | 36.9 ± 1.21 a |
| Isorhamnetin hexoside II | 1.57 ± 0.10 b | 1.61 ± 0.09 b | 1.27 ± 0.07 c | 1.73 ± 0.09 a |
| Dicaffeoylquinic acid isomer | 0.26 ± 0.04 a | 0.28 ± 0.05 a | 0.23 ± 0.03 a | 0.23 ± 0.05 a |
| Feruloylcaffeoylquinic acid | 0.29 ± 0.05 a | 0.30 ± 0.06 a | 0.15 ± 0.03 b | 0.25 ± 0.04 a |
| Tricaffeoylquinic acid | 0.86 ± 0.08 a | 0.79 ± 0.06 a | 0.15 ± 0.02 c | 0.60 ± 0.06 b |
| Luteolin | 3.33 ± 0.15 a | 3.17 ± 0.11 a | 1.78 ± 0.09 c | 2.57 ± 0.10 b |
| Quercetin | 0.89 ± 0.06 a | 0.86 ± 0.06 a | 0.50 ± 0.09 b | 0.35 ± 0.05 c |
| Methoxyquercetin | 0.83 ± 0.08 a | 0.80 ± 0.07 a | 0.61 ± 0.06 b | 0.75 ± 0.07 a |
| Apigenin | 0.39 ± 0.04 a | 0.39 ± 0.05 ab | 0.12 ± 0.01 c | 0.31 ± 0.03 b |
| Diosmetin | 0.24 ± 0.02 a | 0.24 ± 0.03 a | 0.16 ± 0.03 b | 0.23 ± 0.03 a |
| Trihydroxy dimethoxyflavone | 0.35 ± 0.03 a | 0.35 ± 0.04 a | 0.18 ± 0.02 b | 0.31 ± 0.03 a |
| Centaureidin | 3.34 ± 0.14 a | 3.30 ± 0.15 a | 2.09 ± 0.13 c | 2.93 ± 0.17 b |
| Methoxyacacetin | 0.23 ± 0.03 a | 0.23 ± 0.02 a | 0.06 ± 0.01 c | 0.20 ± 0.02 a |
| Dihydroxy trimethoxyflavone | 0.34 ± 0.04 a | 0.32 ± 0.05 a | 0.16 ± 0.02 c | 0.20 ± 0.03 b |
| Casticin | 4.18 ± 0.17 a | 4.02 ± 0.14 a | 2.27 ± 0.11 c | 3.62 ± 0.12 b |
| Undigested | Oral | Gastric | Intestinal | ||
|---|---|---|---|---|---|
| TPC 1 | YE | 105 ± 3 a | 96 ± 3 b | 87 ± 2 c | 91 ± 2 b |
| EE | 224 ± 3 a | 214 ± 2 b | 201 ± 3 d | 208 ± 3 c | |
| TEAC value 2 | YE | 0.36 ± 0.01 a | 0.35 ± 0.01 a | 0.20 ± 0.01 b | 0.22 ± 0.04 b |
| EE | 1.12 ± 0.06 a | 1.06 ± 0.03 a | 0.75 ± 0.06 b | 0.83 ± 0.04 b |
| Apical Compartment | Cell Monolayer | Basolateral Compartment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | 2 h | 4 h | 6 h | 2 h | 4 h | 6 h | 2 h | 4 h | 6 h |
| Apigenin derivative | 18.50 ± 0.04 a | 18.30 ± 0.07 b | 17.70 ± 0.10 c | 0.37 ± 0.0 a | 0.37 ± 0.01 a | 0.36 ± 0.01 a | 0.45 ± 0.01 a | 0.46 ± 0.01 a | 0.42 ± 0.01 b |
| Luteolin-7-O-glucoside | 41.64 ± 0.10 a | 40.98 ± 0.30 a | 39.77 ± 0.04 b | 0.39 ± 0.02 a | 0.36 ± 0.01 a | 0.28 ± 0.01 b | 0.25 ± 0.01 a | 0.24 ± 0.01 a | 0.21 ± 0.01 b |
| 3,4-Dicaffeoyl- quinic acid | 14.85 ± 0.10 a | 12.09 ± 0.10 b | 9.18 ± 0.05 c | 0.68 ± 0.01 a | 0.66 ± 0.02 a | n.d. | 0.76 ± 0.01 a | 0.71 ± 0.01 b | n.d. |
| 3,5-Dicaffeoyl- quinic acid | 4.95 ± 0.12 a | 4.26 ± 0.05 b | 2.99 ± 0.07 c | 0.75 ± 0.01 | n.d. | n.d. | 0.71 ± 0.01 a | 0.70 ± 0.01 a | n.d. |
| Apigenin 7-O-glucoside | 13.34 ± 0.06 a | 12.06 ± 0.08 b | 11.58 ± 0.15 c | 0.24 ± 0.01 a | 0.2 ± 0.01 ab | 0.20 ± 0.01 b | 0.14 ± 0.01 | n.d. | n.d. |
| 4,5-Dicaffeoyl- quinic acid | 22.24 ± 0.14 a | 20.74 ± 0.11 b | 16.28 ± 0.11 c | 0.08 ± 0.01 | n.d. | n.d. | 0.26 ± 0.01 a | 0.13 ± 0.01 b | 0.09 ± 0.02 c |
| Apigenin | 0.16 ± 0.01 a | 0.09 ± 0.02 b | n.d. | 0.04 ± 0.01 | n.d. | n.d. | 0.13 ± 0.01 c | 0.18 ± 0.01 b | 0.27 ± 0.01 a |
| Diosmetin | 0.95 ± 0.02 a | 0.94 ± 0.03 a | 0.94 ± 0.01 a | 0.89 ± 0.01 a | 0.66 ± 0.01 b | 0.41 ± 0.01 c | 0.40 ± 0.01 c | 0.79 ± 0.02 b | 1.53 ± 0.11 a |
| Centaureidin | 3.27 ± 0.02 a | 2.18 ± 0.07 b | 0.75 ± 0.01 c | 0.58 ± 0.01 a | 0.41 ± 0.01 b | n.d. | 0.48 ± 0.01 c | 0.84 ± 0.07 b | 1.01 ± 0.02 a |
| Methoxyacacetin | 0.05 ± 0.01 a | n.d. | n.d. | 0.13 ± 0.01 a | 0.10 ± 0.0 b | 0.10 ± 0.01 b | 0.12 ± 0.01 c | 0.17 ± 0.01 b | 0.20 ± 0.02 a |
| Casticin | 5.17 ± 0.11 a | 4.44 ± 0.09 b | 3.73 ± 0.07 c | 1.87 ± 0.01 a | 1.53 ± 0.01 b | 1.08 ± 0.01 c | 1.77 ± 0.02 c | 2.3 ± 0.02 b | 3.43 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalva, M.; Jaime, L.; Siles-Sánchez, M.d.l.N.; Santoyo, S. Bioavailability Assessment of Yarrow Phenolic Compounds Using an In Vitro Digestion/Caco-2 Cell Model: Anti-Inflammatory Activity of Basolateral Fraction. Molecules 2022, 27, 8254. https://doi.org/10.3390/molecules27238254
Villalva M, Jaime L, Siles-Sánchez MdlN, Santoyo S. Bioavailability Assessment of Yarrow Phenolic Compounds Using an In Vitro Digestion/Caco-2 Cell Model: Anti-Inflammatory Activity of Basolateral Fraction. Molecules. 2022; 27(23):8254. https://doi.org/10.3390/molecules27238254
Chicago/Turabian StyleVillalva, Marisol, Laura Jaime, María de las Nieves Siles-Sánchez, and Susana Santoyo. 2022. "Bioavailability Assessment of Yarrow Phenolic Compounds Using an In Vitro Digestion/Caco-2 Cell Model: Anti-Inflammatory Activity of Basolateral Fraction" Molecules 27, no. 23: 8254. https://doi.org/10.3390/molecules27238254
APA StyleVillalva, M., Jaime, L., Siles-Sánchez, M. d. l. N., & Santoyo, S. (2022). Bioavailability Assessment of Yarrow Phenolic Compounds Using an In Vitro Digestion/Caco-2 Cell Model: Anti-Inflammatory Activity of Basolateral Fraction. Molecules, 27(23), 8254. https://doi.org/10.3390/molecules27238254