PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells
Abstract
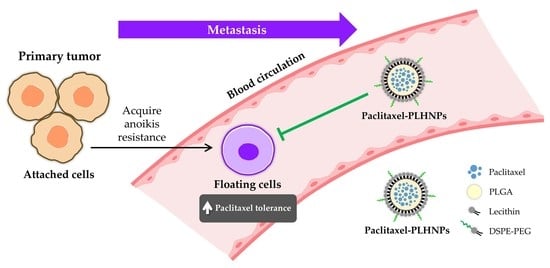
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Paclitaxel-PLHNPs
2.2. Evaluation of Anoikis Resistance
2.3. Comparison of the Cytotoxic Effect of Paclitaxel-PLHNPs and Free Drug in Anoikis-Resistant A549 Cells
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Nanoparticle Preparation and Characterization
4.3. Cell Culture
4.4. Caspase-3 Activity Assay
4.5. Cytotoxicity Assay
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Adeshakin, F.O.; Adeshakin, A.O.; Afolabi, L.O.; Yan, D.; Zhang, G.; Wan, X. Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Front. Oncol. 2021, 11, 626577. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012, 226, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, P.; Giannoni, E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008, 76, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Kahraman, D.C.; Ergun, S.G.; Cetin-Atalay, R.; Baytas, S.N. Synthesis of novel indole-isoxazole hybrids and evaluation of their cytotoxic activities on hepatocellular carcinoma cell lines. BMC Chem. 2021, 15, 66. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for locally advanced and metastatic non–small cell lung cancer: A review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and future development in lung cancer diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Bodor, J.N.; Kasireddy, V.; Borghaei, H. First-line therapies for metastatic lung adenocarcinoma without a driver mutation. J. Oncol. Pract. 2018, 14, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in modern oncology and nanomedicine-based cancer therapy. Oxid. Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.S.; Sharma, A.; Kumari, R.; Mohammad, N.; Singh, S.V.; Bhat, M.K. Inherent and acquired resistance to paclitaxel in hepatocellular carcinoma: Molecular events involved. PLoS ONE 2013, 8, e61524. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.K.; Fossella, F.V.; Winn, R.J.; Shin, D.M.; Hynes, H.E.; Gross, H.M.; Davilla, E.; Leimert, J.; Dhingra, H.; Raber, M.N.; et al. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 1993, 85, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Kim, K.; Glick, J.; Anderson, T.; Karp, D.; Johnson, D. Phase II Study of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer: The eastern cooperative oncology group results. J. Natl. Cancer Inst. 1993, 85, 388–394. [Google Scholar] [CrossRef]
- Atjanasuppat, K.; Lirdprapamongkol, K.; Jantaree, P.; Svasti, J. Non-adherent culture induces paclitaxel resistance in H460 lung cancer cells via ERK-mediated up-regulation of βIVa-tubulin. Biochem. Biophys. Res. Commun. 2015, 466, 493–498. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, S.; Ni, S.; Zhang, B.; Kung, A.C.F.; Gao, J.; Lu, A.; Zhang, G. targeting strategies for enhancing paclitaxel specificity in chemotherapy. Front. Cell Dev. Biol. 2021, 9, 626910. [Google Scholar] [CrossRef]
- Raza, F.; Zafar, H.; Khan, M.W.; Ullah, A.; Khan, A.U.; Baseer, A.; Fareed, R.; Sohail, M. Recent advances in the targeted delivery of paclitaxel nanomedicine for cancer therapy. Mater. Adv. 2022, 3, 2268–2290. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Eid, A.M.; Abubaker, A.; Mufleh, O.; Al-Hroub, Q.; Sobuh, S. Synthesis of novel isoxazole–carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Hawash, M. Biological evaluation of safrole oil and safrole oil nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complement. Med. Ther. 2021, 21, 159. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar]
- Tanaka, M.; Hattori, Y.; Ishii, T.; Tohnai, R.; Itoh, S.; Kawa, Y.; Kono, Y.; Urata, Y.; Satouchi, M. The efficacy of carboplatin plus nanoparticle albumin-bound paclitaxel after cisplatin plus pemetrexed in non-squamous non-small-cell lung cancer patients. Respir. Investig. 2020, 58, 269–274. [Google Scholar] [CrossRef]
- Nadaf, S.J.; Killedar, S.G.; Kumbar, V.M.; Bhagwat, D.A.; Gurav, S.S. Pazopanib-laden lipid based nanovesicular delivery with augmented oral bioavailability and therapeutic efficacy against non-small cell lung cancer. Int. J. Pharm. 2022, 628, 122287. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Multifunctional lipid-based nanoparticles for codelivery of anticancer drugs and siRNA for treatment of non-small cell lung cancer with different level of resistance and EGFR mutations. Pharmaceutics 2021, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, A.; Tu, H.; Huang, C.; Wang, H.; Yu, Z.; Wang, X.; Cao, L.; Shu, Y.; Wang, H.; et al. Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: An open-label, randomized, multicenter, phase III trial. Ann. Oncol. 2021, 32, 85–96. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, J.; El-Hammadi, M.M.; Ortiz, R.; Cayero-Otero, M.D.; Cabeza, L.; Perazzoli, G.; Martin-Banderas, L.; Baeyens, J.M.; Prados, J.; Melguizo, C. A novel nanoformulation of PLGA with high non-ionic surfactant content improves in vitro and in vivo PTX activity against lung cancer. Pharmacol. Res. 2019, 141, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ji, W.; Chen, S.; Bao, Y.; Tan, S.; Lu, S.; Wu, K.; Chu, Q. A novel paclitaxel-loaded poly(d,l-lactide-co-glycolide)-Tween 80 copolymer nanoparticle overcoming multidrug resistance for lung cancer treatment. Int. J. Nanomed. 2016, 11, 2119–2131. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloids Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- De, S.; Robinson, D.H. Particle size and temperature effect on the physical stability of PLGA nanospheres and microspheres containing Bodipy. AAPS PharmSciTech 2004, 5, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, Y.; Takeuchi, T.; Nakamura, Y.; Yoshihara, M.; Matsukuma, S.; Nakayama, H.; Ohgane, N.; Yokose, T.; Kameda, Y.; Tsuchiya, E.; et al. Lung adenocarcinoma cells floating in lymphatic vessels resist anoikis by expressing phosphorylated Src. J. Pathol. 2010, 220, 574–585. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Pramual, S.; Lirdprapamongkol, K.; Jouan-Hureaux, V.; Barberi-Heyob, M.; Frochot, C.; Svasti, J.; Niamsiri, N. Overcoming the diverse mechanisms of multidrug resistance in lung cancer cells by photodynamic therapy using pTHPP-loaded PLGA-lipid hybrid nanoparticles. Eur. J. Pharm. Biopharm. 2020, 149, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, R.Z.; Duan, Z.; Lamendola, D.E.; Penson, R.T.; Seiden, M.V. Paclitaxel resistance: Molecular mechanisms and pharmacologic manipulation. Curr. Cancer Drug Targets 2003, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kars, M.D.; Işeri, Ö.D.; Gündüz, U. A microarray based expression profiling of paclitaxel and vincristine resistant MCF-7 cells. Eur. J. Pharmacol. 2011, 657, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.; Kim, J.B.; Kim, J.; Kim, H. Entrapped doxorubicin nanoparticles for the treatment of metastatic anoikis-resistant cancer cells. Cancer Lett. 2013, 332, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiri, N. Polymer-lipid-PEG hybrid nanoparticles as photosensitizer carrier for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2017, 173, 12–22. [Google Scholar] [CrossRef] [PubMed]





| Size | PDI | Zeta Potential | |
|---|---|---|---|
| Empty PLHNPs | 0.09 | ||
| Paclitaxel-PLHNPs | 0.11 |
| IC50 at 72 h | Fold Resistance | ||
|---|---|---|---|
| A549 Attached Cells | A549 Floating Cells | ||
| Free paclitaxel | 53.1 | ||
| Paclitaxel-PLHNPs | 10.3 | ||
| Fold change in IC50 | 71.6-fold decreased | 370.4-fold decreased | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pramual, S.; Lirdprapamongkol, K.; Atjanasuppat, K.; Chaisuriya, P.; Niamsiri, N.; Svasti, J. PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells. Molecules 2022, 27, 8295. https://doi.org/10.3390/molecules27238295
Pramual S, Lirdprapamongkol K, Atjanasuppat K, Chaisuriya P, Niamsiri N, Svasti J. PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells. Molecules. 2022; 27(23):8295. https://doi.org/10.3390/molecules27238295
Chicago/Turabian StylePramual, Sasivimon, Kriengsak Lirdprapamongkol, Korakot Atjanasuppat, Papada Chaisuriya, Nuttawee Niamsiri, and Jisnuson Svasti. 2022. "PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells" Molecules 27, no. 23: 8295. https://doi.org/10.3390/molecules27238295
APA StylePramual, S., Lirdprapamongkol, K., Atjanasuppat, K., Chaisuriya, P., Niamsiri, N., & Svasti, J. (2022). PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells. Molecules, 27(23), 8295. https://doi.org/10.3390/molecules27238295