Self-Assembled Monolayers of Molecular Conductors with Terpyridine-Metal Redox Switching Elements: A Combined AFM, STM and Electrochemical Study
Abstract
:1. Introduction
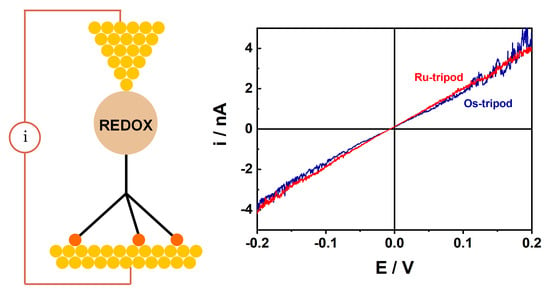
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. SAM Preparation
3.3. Electrochemistry
3.4. AFM and STM Measurements
3.5. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sauvage, J.-P.; Collin, J.-P.; Chambron, J.-C.; Guillerez, S.; Coudret, C. Ruthenlum(II) and Osmium(II) Bis(terpyridine) Complexes in Covalently-Linked Multicomponent Systems: Synthesis, Electrochemical Behavior, Absorption Spectra, and Photochemical and Photophysical Properties. Chem. Rev. 1994, 94, 993–1019. [Google Scholar] [CrossRef]
- Baranoff, E.; Collin, J.-P.; Flamigni, L.; Sauvage, J.-P. From ruthenium(II) to iridium(III): 15 years of triads based on bis-terpyridine complexes. Chem. Soc. Rev. 2004, 33, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X. Molecular-Scale Electronics: From Concept to Function. Chem. Rev. 2016, 116, 4318–4440. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Small, J.P.; Klare, J.E.; Wang, Y.; Purewal, M.S.; Tam, I.W.; Hong, B.H.; Caldwell, R.; Huang, L.; O’Brien, S. Covalently Bridging Gaps in Single-Walled Carbon Nanotubes with Conducting Molecules. Science 2006, 311, 356–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, E.A.; Moth-Poulsen, K.; van der Zant, H.S.J.; Paaske, J.; Hedegård, P.; Flensberg, K.; Bendix, J.; Bjørnholm, T. Electrical manipulation of spin states in a single electrostatically gated transition-metal complex. Nano Lett. 2010, 10, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, R.; Wu, K.-H.; Matsuoka, R.; Maeda, H.; Nishihara, H. π-Conjugated bis(terpyridine)metal complex molecular wires. Chem. Soc. Rev. 2015, 44, 7698–7714. [Google Scholar] [CrossRef]
- Sakamoto, R.; Ohirabaru, Y.; Matsuoka, R.; Maeda, H.; Katagiri, S.; Nishihara, H. Orthogonal bis(terpyridine)–Fe(II) metal complex oligomer wires on a tripodal scaffold: Rapid electron transport. Chem. Commun. 2013, 49, 7108–7110. [Google Scholar] [CrossRef]
- Wu, K.-H.; Sakamoto, R.; Maeda, H.; Jia Han Phua, E.; Nishihara, H. Ultralong π-Conjugated Bis(terpyridine)metal Polymer Wires Covalently Bound to a Carbon Electrode: Fast Redox Conduction and Redox Diode Characteristics. Molecules 2021, 26, 4267. [Google Scholar] [CrossRef]
- Maeda, H.; Sakamoto, R.; Nishihara, H. Interfacial synthesis of electrofunctional coordination nanowires and nanosheets of bis(terpyridine) complexes. Coord. Chem. Rev. 2017, 346, 139–149. [Google Scholar] [CrossRef]
- Ozawa, H.; Baghernejad, M.; Al-Owaedi, O.A.; Kaliginedi, V.; Nagashima, T.; Ferrer, J.; Wandlowski, T.; García-Suárez, V.M.; Broekmann, P.; Lambert, C.J.; et al. Synthesis and Single-Molecule Conductance Study of Redox-Active Ruthenium Complexes with Pyridyl and Dihydrobenzo[b]thiophene Anchoring Groups. Chem. Eur. J. 2016, 22, 12732–12740. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kato, Y.; Sugimoto, K.; Kawano, R.; Tada, T.; Fujii, S.; Kiguchi, M.; Akita, M. Single-molecule junctions of multinuclear organometallic wires: Long-range carrier transport brought about by metal–metal interaction. Chem. Sci. 2021, 12, 4338–4344. [Google Scholar] [CrossRef] [PubMed]
- Mennicken, M.; Peter, S.K.; Kaulen, C.; Simon, U.; Karthäuser, S. Impact of device design on the electronic and optoelectronic properties of integrated Ru-terpyridine complexes. Beilstein J. Nanotechnol. 2022, 13, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Valášek, M.; Homberg, J.; Edelmann, K.; Gerhard, L.; Wulfhekel, W.; Fuhr, O.; Wächter, T.; Zharnikov, M.; Kolivoška, V.; et al. Importance of the anchor group position (Para ver-sus Meta) in tetraphenylmethane tripods: Synthesis and self-assembly features. Chem. Eur. J. 2016, 22, 13218–13235. [Google Scholar] [CrossRef] [PubMed]
- Sebechlebská, T.; Šebera, J.; Kolivoška, V.; Lindner, M.; Gasior, J.; Mészáros, G.; Valášek, M.; Mayor, M.; Hromadová, M. Investigation of the geometrical arrangement and single molecule charge transport in self-assembled monolayers of molecular towers based on tetraphenylmethane tripod. Electrochim. Acta 2017, 258, 1191–1200. [Google Scholar] [CrossRef]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 14–31. [Google Scholar] [CrossRef]
- Higgins, S.J.; Nichols, R.J. Metal/molecule/metal junction studies of organometallic and coordination complexes; What can transition metals do for molecular electronics? Polyhedron 2018, 140, 25–34. [Google Scholar] [CrossRef]
- Bu, D.; Xiong, Y.; Tan, Y.N.; Meng, M.; Low, P.J.; Kuang, D.-B.; Liu, C.Y. Understanding the charge transport properties of redox active metal–organic conjugated wires. Chem. Sci. 2018, 9, 3438–3450. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, Q.; Bragança, A.M.; Alcácer, L.; Morgado, J. Conductance of Well-Defined Porphyrin Self-Assembled Molecular Wires up to 14 nm in Length. J. Phys. Chem. C 2014, 118, 7229–7234. [Google Scholar] [CrossRef]
- Sakamoto, R.; Katagiri, S.; Maeda, H.; Nishimori, Y.; Miyashita, S.; Nishihara, H. Electron Transport Dynamics in Redox-Molecule-Terminated Branched Oligomer Wires on Au(111). J. Am. Chem. Soc. 2015, 137, 734–741. [Google Scholar] [CrossRef]
- Tuccitto, N.; Ferri, V.; Cavazzini, M.; Quici, S.; Zhavnerko, G.; Licciardello, A.; Rampi, M.A. Highly conductive ~40-nm-long molecular wires assembled by stepwise incorporation of metal centres. Nat. Mater. 2009, 8, 41–46. [Google Scholar] [CrossRef]
- Maeda, H.; Sakamoto, R.; Nishihara, H. Rapid Electron Transport Phenomenon in the Bis(terpyridine) Metal Complex Wire: Marcus Theory and Electrochemical Impedance Spectroscopy Study. J. Phys. Chem. Lett. 2015, 6, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Ryota, S.; Shunsuke, K.; Hiroaki, M.; Hiroshi, N. Triarylamine-conjugated Bis(terpyridine)–Iron(II) Complex Wires: Rapid and Long-range Electron-transport Ability. Chem. Lett. 2013, 42, 553–555. [Google Scholar]
- Musumeci, C.; Zappalà, G.; Martsinovich, N.; Orgiu, E.; Schuster, S.; Quici, S.; Zharnikov, M.; Troisi, A.; Licciardello, A.; Samorì, P. Nanoscale Electrical Investigation of Layer-by-Layer Grown Molecular Wires. Adv. Mater. 2014, 26, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, G.; Esdaile, L.J.; Anderson, H.L.; Martin, S.; Bethell, D.; Higgins, S.J.; Nichols, R.J. Comparison of the Conductance of Three Types of Porphyrin-Based Molecular Wires: β,meso,β-Fused Tapes, meso-Butadiyne-Linked and Twisted meso-meso Linked Oligomers. Adv. Mater. 2012, 24, 653–657. [Google Scholar] [CrossRef]
- Sedghi, G.; García-Suárez, V.M.; Esdaile, L.J.; Anderson, H.L.; Lambert, C.J.; Martín, S.; Bethell, D.; Higgins, S.J.; Elliott, M.; Bennett, N.; et al. Long-range electron tunnelling in oligo-porphyrin molecular wires. Nat. Nanotechnol. 2011, 6, 517–523. [Google Scholar] [CrossRef]
- Bruce, R.C.; Wang, R.; Rawson, J.; Therien, M.J.; You, W. Valence Band Dependent Charge Transport in Bulk Molecular Electronic Devices Incorporating Highly Conjugated Multi-[(Porphinato)Metal] Oligomers. J. Am. Chem. Soc. 2016, 138, 2078–2081. [Google Scholar] [CrossRef]
- Li, Z.; Park, T.-H.; Rawson, J.; Therien, M.J.; Borguet, E. Quasi-Ohmic Single Molecule Charge Transport through Highly Conjugated meso-to-meso Ethyne-Bridged Porphyrin Wires. Nano Lett. 2012, 12, 2722–2727. [Google Scholar] [CrossRef]
- Figgemeier, E.; Merz, L.; Hermann, B.A.; Zimmermann, Y.C.; Housecroft, C.E.; Güntherodt, H.-J.; Constable, E.C. Self-Assembled Monolayers of Ruthenium and Osmium Bis-Terpyridine Complexes–Insights of the Structure and Interaction Energies by Combining Scanning Tunneling Microscopy and Electrochemistry. J. Phys. Chem. B 2003, 107, 1157–1162. [Google Scholar] [CrossRef]
- Šebera, J.; Kolivoška, V.; Valášek, M.; Gasior, J.; Sokolová, R.; Mészáros, G.; Hong, W.; Mayor, M.; Hromadová, M. Tuning Charge Transport Properties of Asymmetric Molecular Junctions. J. Phys. Chem. C 2017, 121, 12885–12894. [Google Scholar] [CrossRef]
- Valášek, M.; Lindner, M.; Mayor, M. Rigid multipodal platforms for metal surface. Beilstein J. Nanotechnol. 2016, 7, 374–405. [Google Scholar] [CrossRef] [Green Version]
- Valášek, M.; Mayor, M. Spatial and Lateral Control of Functionality by Rigid Molecular Platforms. Chem. Eur. J. 2017, 23, 13538–13548. [Google Scholar] [CrossRef] [PubMed]
- Kolivoška, V.; Šebera, J.; Sebechlebská, T.; Lindner, M.; Gasior, J.; Mészáros, G.; Mayor, M.; Valášek, M.; Hromadová, M. Probabilistic mapping of single molecule junction configurations as a tool to achieve desired geometry of asymmetric tripodal molecules. Chem. Commun. 2019, 55, 3351–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šebera, J.; Lindner, M.; Gasior, J.; Meszáros, G.; Fuhr, O.; Mayor, M.; Valášek, M.; Kolivoška, V.; Hromadová, M. Tuning contact conductance of anchoring groups in single molecule junctions by molecular design. Nanoscale 2019, 11, 12959–12964. [Google Scholar] [CrossRef] [PubMed]
- Nováková Lachmanová, Š.; Vavrek, F.; Sebechlebská, T.; Kolivoška, V.; Valášek, M.; Hromadová, M. Charge transfer in self-assembled monolayers of molecular conductors containing tripodal anchor and terpyridine-metal redox switching element. Electrochim. Acta 2021, 384, 138302. [Google Scholar] [CrossRef]
- Yang, G.; Liu, G. New Insights for Self-Assembled Monolayers of Organothiols on Au(111) Revealed by Scanning Tunneling Microscopy. J. Phys. Chem. B 2003, 107, 8746–8759. [Google Scholar] [CrossRef]
- Palmer, R.E.; Guo, Q. Imaging thin films of organic molecules with the scanning tunnelling microscope. Phys. Chem. Chem. Phys. 2002, 4, 4275–4284. [Google Scholar] [CrossRef]
- Petrov, E.G. Superexchange Nonresonant Tunneling Current across a Molecular Wire. JETP Lett. 2018, 108, 302–311. [Google Scholar] [CrossRef]
- Simmons, J.G. Generalized Formula for the Electric Tunnel Effect between Similar Electrodes Separated by a Thin Insulating Film. J. Appl. Phys. 1963, 34, 1793–1803. [Google Scholar] [CrossRef] [Green Version]
- Finklea, H.O.; Hanshew, D.D. Electron-Transfer Kinetics in Organized Thiol Monolayers with Attached Pentaammine(pyridine)ruthenium Redox Centers. J. Am. Chem. Soc. 1992, 114, 3173–3181. [Google Scholar] [CrossRef]
- Nováková Lachmanová, Š.; Kolivoška, V.; Šebera, J.; Gasior, J.; Mészáros, G.; Dupeyre, G.; Lainé, P.P.; Hromadová, M. Environmental Control of Single-Molecule Junction Evolution and Conductance: A Case Study of Expanded Pyridinium Wiring. Angew. Chem. Int. Ed. 2021, 60, 4732–4739. [Google Scholar] [CrossRef]
- Duncan, T.V.; Ishizuka, T.; Therien, M.J. Molecular engineering of in-tensely near-infrared absorbing excited states in highly conjugated oligo(porphinato)zinc−(Polypyridyl)metal(II) supermolecules. J. Am. Chem. Soc. 2007, 129, 9691–9703. [Google Scholar] [CrossRef] [PubMed]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Pobelov, I.V. Electron Transport Studies—An Electrochemical Scanning Tunneling Microscopy Approach. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2008. [Google Scholar]
- Gwyddion—Free SPM Data Analysis Software. Available online: http://gwyddion.net/ (accessed on 6 September 2022).
- Becke, A.D. Density-Functional Thermochemistry. 3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Řezáč, J.; Hobza, P. Advanced Corrections of Hydrogen Bonding and Dispersion for Semiempirical Quantum Mechanical Methods. J. Chem. Theory Comput. 2012, 8, 141–151. [Google Scholar] [CrossRef] [PubMed]









| Setpoint Current * | 0.1 nA | 0.2 nA | 0.5 nA | 1.0 nA |
|---|---|---|---|---|
| Ru-tripod SAM | 552.0 ± 73.1 MΩ 1.81 ± 0.24 nS | 271.8 ± 31.6 MΩ 3.68 ± 0.43 nS | 111.7 ± 8.2 MΩ 8.95 ± 0.65 nS | 53.9 ± 5.5 MΩ 18.6 ± 1.9 nS |
| Os-tripod SAM | 557.0 ± 74.6 MΩ 1.79 ± 0.24 nS | 281.6 ± 19.3 MΩ 3.55 ± 0.24 nS | 114.0 ± 9.5 MΩ 8.77 ± 0.73 nS | 57.0 ± 4.3 MΩ 17.5 ± 1.3 nS |
| System | Attenuation Factor | |
|---|---|---|
| Before CV | After CV | |
| Ru-tripod SAM | (0.693 ± 0.012) nm−1 | (0.668 ± 0.007) nm−1 |
| Os-tripod SAM | (0.468 ± 0.005) nm−1 | (0.843 ± 0.021) nm−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocábová, J.; Vavrek, F.; Nováková Lachmanová, Š.; Šebera, J.; Valášek, M.; Hromadová, M. Self-Assembled Monolayers of Molecular Conductors with Terpyridine-Metal Redox Switching Elements: A Combined AFM, STM and Electrochemical Study. Molecules 2022, 27, 8320. https://doi.org/10.3390/molecules27238320
Kocábová J, Vavrek F, Nováková Lachmanová Š, Šebera J, Valášek M, Hromadová M. Self-Assembled Monolayers of Molecular Conductors with Terpyridine-Metal Redox Switching Elements: A Combined AFM, STM and Electrochemical Study. Molecules. 2022; 27(23):8320. https://doi.org/10.3390/molecules27238320
Chicago/Turabian StyleKocábová, Jana, František Vavrek, Štěpánka Nováková Lachmanová, Jakub Šebera, Michal Valášek, and Magdaléna Hromadová. 2022. "Self-Assembled Monolayers of Molecular Conductors with Terpyridine-Metal Redox Switching Elements: A Combined AFM, STM and Electrochemical Study" Molecules 27, no. 23: 8320. https://doi.org/10.3390/molecules27238320
APA StyleKocábová, J., Vavrek, F., Nováková Lachmanová, Š., Šebera, J., Valášek, M., & Hromadová, M. (2022). Self-Assembled Monolayers of Molecular Conductors with Terpyridine-Metal Redox Switching Elements: A Combined AFM, STM and Electrochemical Study. Molecules, 27(23), 8320. https://doi.org/10.3390/molecules27238320