Natural Products as Anticancer Agents: Current Status and Future Perspectives
Abstract
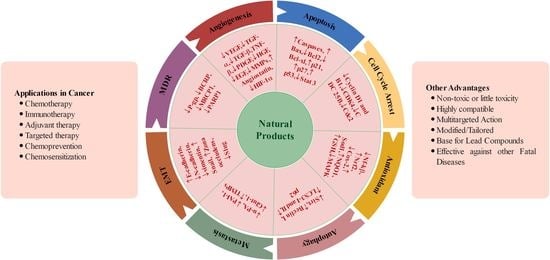
:1. Introduction
2. Natural Products (NPs) against Cancer
2.1. Natural Products against Chemoresistance
2.2. Natural Products against Metastasis
2.3. Natural Products and Cancer Immunotherapy
2.4. Natural Products in Combination with Other Chemotherapeutic Drugs
3. Natural Products against Some Common Types of Cancer
3.1. Lung Cancer
3.2. Breast Cancer
3.3. Ovarian Cancer
3.4. Colon Cancer
3.5. Brain Cancer
3.6. Liver Cancer
3.7. Head and Neck Cancer
3.8. Prostate Cancer
3.9. Hematological Cancer
3.10. Miscellaneous Cancer
4. Novel Formulations of Natural Products for Chemotherapy
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cadoná, F.C.; Dantas, R.F.; de Mello, G.H.; Silva, F.P., Jr. Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+)-catechin. Crit. Rev. Food Sci. Nutr. 2021, 62, 7222–7241. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.; Davey-Smith, G. Identifying Novel Causes of Cancers to Enhance Cancer Prevention: New Strategies Are Needed. JNCI J. Natl. Cancer Inst. 2022, 114, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, M.T.; Tumpa, M.A.A.; Yamin, M.; Islam, T.; Park, M.N.; Islam, M.R.; Rauf, A.; Sharma, R.; Cavalu, S. Exploring the recent trends in perturbing the cellular signaling pathways in cancer by natural products. Front. Pharmacol. 2022, 13, 950109. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, Y. Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives. Drug Discov. Today 2022, 27, 1936–1944. [Google Scholar] [CrossRef]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The role of natural products and their multitargeted approach to treat solid cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar]
- Liu, S.; Khan, A.R.; Yang, X.; Dong, B.; Ji, J.; Zhai, G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release 2021, 335, 1–20. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [Green Version]
- Bashraheel, S.S.; Domling, A.; Goda, S.K. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. Biomed. Pharmacother. 2020, 125, 110009. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef]
- Nonnekens, J.; Hoeijmakers, J.H. After surviving cancer, what about late life effects of the cure? EMBO Mol. Med. 2017, 9, 4–6. [Google Scholar] [CrossRef]
- Wigmore, P.M.; Mustafa, S.; El-Beltagy, M.; Lyons, L.; Umka, J.; Bennett, G. Effects of 5-FU. In Chemo Fog; Springer: New York, NY, USA, 2010; pp. 157–164. [Google Scholar]
- Cardona-Mendoza, A.; Olivares-Niño, G.; Díaz-Báez, D.; Lafaurie, G.I.; Perdomo, S.J. Chemopreventive and Anti-tumor Potential of Natural Products in Oral Cancer. Nutr. Cancer 2022, 74, 779–795. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef]
- Pepper, J.W.; Findlay, C.S.; Kassen, R.; Spencer, S.L.; Maley, C.C. Synthesis: Cancer research meets evolutionary biology. Evol. Appl. 2009, 2, 62–70. [Google Scholar] [CrossRef]
- Yagüe, E.; Arance, A.; Kubitza, L.; O’Hare, M.; Jat, P.; Ogilvie, C.M.; Hart, I.R.; Higgins, C.F.; Raguz, S. Ability to acquire drug resistance arises early during the tumorigenesis process. Cancer Res. 2007, 67, 1130–1137. [Google Scholar]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from January 1981 to September 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Satheeshkumar, P.K. 5-Natural Products From Plants: Recent Developments in Phytochemicals, Phytopharmaceuticals, and Plant-Based Neutraceuticals as Anticancer Agents. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 145–163. [Google Scholar]
- Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T. Berberine in human oncogenic herpesvirus infections and their linked cancers. Viruses 2021, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lachance, H.; Wetzel, S.; Kumar, K.; Waldmann, H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 2012, 55, 5989–6001. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Ramos, A.C.; Peláez, R.; López, J.L.; Caballero, E.; Medarde, M.; San Feliciano, A. Heterolignanolides. Furo-and thieno-analogues of podophyllotoxin and thuriferic acid. Tetrahedron 2001, 57, 3963–3977. [Google Scholar] [CrossRef]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef]
- Găman, A.M.; Egbuna, C.; Găman, M.-A. Natural Bioactive Lead Compounds Effective Against Haematological Malignancies. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–115. [Google Scholar]
- Wang, H.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.; Yang, C.; Li, Y.; Shao, M.; Su, W.; Song, N. Curcumin regulates cancer progression: Focus on ncRNAs and molecular signaling pathways. Front. Oncol. 2021, 11, 660712. [Google Scholar] [CrossRef]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in Cancer Cell Chemosensitization: Current Knowledge and Future Perspectives. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- Monisha, J.; Jaiswal, A.; Banik, K.; Choudhary, H.; Singh, A.K.; Bordoloi, D.; Kunnumakkara, A.B. Cancer Cell Chemoresistance: A Prime Obstacle in Cancer Therapy. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 15–49. [Google Scholar]
- Maurya, S.K.; Shadab, G.; Siddique, H.R. Chemosensitization of Therapy Resistant Tumors: Targeting Multiple Cell Signaling Pathways by Lupeol, A Pentacyclic Triterpene. Curr. Pharm. Des. 2020, 26, 455–465. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, B.; Chen, X. Induction of Programmed Necrosis by Phytochemicals in Colorectal Cancer. In Drug Resistance in Colorectal Cancer: Molecular Mechanisms and Therapeutic Strategies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–133. [Google Scholar]
- Hsu, Y.-H.; Chen, S.-Y.; Wang, S.-Y.; Lin, J.-A.; Yen, G.-C. Pterostilbene Enhances Cytotoxicity and Chemosensitivity in Human Pancreatic Cancer Cells. Biomolecules 2020, 10, 709. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, D. EGCG maintained Nrf2-mediated redox homeostasis and minimized etoposide resistance in lung cancer cells. J. Funct. Foods 2019, 62, 103553. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef]
- Lan, C.-Y.; Chen, S.-Y.; Kuo, C.-W.; Lu, C.-C.; Yen, G.-C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019, 27, 887–896. [Google Scholar] [CrossRef]
- Muthusamy, G.; Gunaseelan, S.; Prasad, N.R. Ferulic acid reverses P-glycoprotein-mediated multidrug resistance via inhibition of PI3K/Akt/NF-κB signaling pathway. J. Nutr. Biochem. 2019, 63, 62–71. [Google Scholar] [CrossRef]
- Irani, S. Emerging insights into the biology of metastasis: A review article. Iran. J. Basic Med. Sci. 2019, 22, 833–847. [Google Scholar]
- Kapinova, A.; Kubatka, P.; Liskova, A.; Baranenko, D.; Kruzliak, P.; Matta, M.; Büsselberg, D.; Malicherova, B.; Zulli, A.; Kwon, T.K.; et al. Controlling metastatic cancer: The role of phytochemicals in cell signaling. J. Cancer Res. Clin. Oncol. 2019, 145, 1087–1109. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, Y.; Pan, Y.; Ding, X.; Rhea, P.; Ding, J.; Hawke, D.H.; Felsher, D.; Narla, G.; Lu, Z.; et al. Mistletoe extract Fraxini inhibits the proliferation of liver cancer by down-regulating c-Myc expression. Sci. Rep. 2019, 9, 6428. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal. 2020, 28, 14–37. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Chen, B.; Jiang, R.; Li, J.; Wang, B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell. Immunol. 2017, 311, 86–93. [Google Scholar] [CrossRef]
- Loo, W.T.; Jin, L.; Chow, L.W.; Cheung, M.N.; Wang, M. Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expert Opin. Investig. Drugs 2010, 19, S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vacchelli, E.; Eggermont, A.; Fridman, W.H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology 2012, 1, 1111–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.T.; Lai, T.H.; Chyan, Y.J.; Yin, S.Y.; Chen, Y.H.; Wei, W.C.; Yang, N.-S. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS ONE 2015, 10, e0122374. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Zeng, Z.; Chen, M.Q. Roles of Kupffer cells in liver transplantation. Hepato-Gastroenterology 2012, 59, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xiao, W.; Tian, Z. NK Cell-Based Immunotherapy for Cancer. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2017; pp. 37–54. [Google Scholar]
- Wu, X.-T.; Liu, J.-Q.; Lu, X.-T.; Chen, F.-X.; Zhou, Z.-H.; Wang, T.; Zhu, S.-P.; Fei, S.-J. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int. Immunopharmacol. 2013, 16, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Wang, D.; Ma, X.; Chen, W.; Guo, S.; Guan, H. Effects of total flavonoids of sea buckthorn (Hippophae rhamnoides L.) on cytotoxicity of NK92-MI cells. Int. J. Immunopathol. Pharmacol. 2017, 30, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Epling-Burnette, P.K.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-T.; Li, J.; Qi, X.; Pei, Y.-x.; Shi, W.-G.; Lin, H.-S. Effects of Shugan Jianpi Formula (疏肝健脾方) on myeloid-derived suppression cells-mediated depression breast cancer mice. Chin. J. Integr. Med. 2017, 23, 453–460. [Google Scholar] [CrossRef]
- Murakami, H.; Ogawara, H.; Hiroshi, H. Th1/Th2 cells in patients with multiple myeloma. Hematology 2004, 9, 41–45. [Google Scholar] [CrossRef]
- Wei, H.; Sun, R.; Xiao, W.; Feng, J.; Zhen, C.; Xu, X.; Tian, Z. Type two cytokines predominance of human lung cancer and its reverse by traditional Chinese medicine TTMP. Cell. Mol. Immunol. 2004, 1, 63–70. [Google Scholar]
- Zhang, M.-Y.; Guo, J.; Hu, X.-M.; Zhao, S.-Q.; Li, S.-L.; Wang, J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019, 10, 4361–4371. [Google Scholar] [CrossRef]
- Takei, M.; Tachikawa, E.; Hasegawa, H.; Lee, J.-J. Dendritic cells maturation promoted by M1 and M4, end products of steroidal ginseng saponins metabolized in digestive tracts, drive a potent Th1 polarization. Biochem. Pharmacol. 2004, 68, 441–452. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.-L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef]
- Li, Q.; Bao, J.-M.; Li, X.-L.; Zhang, T.; Shen, X.-H. Inhibiting effect of Astragalus polysaccharides on the functions of CD4+ CD25highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin. Med. J. 2012, 125, 786–793. [Google Scholar]
- Du, X.; Chen, X.; Zhao, B.; Lv, Y.; Zhang, H.; Liu, H.; Chen, Z.; Chen, Y.; Zeng, X. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunol. Med. Microbiol. 2011, 63, 228–235. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Liu, B.; Xu, L.; Zhao, H.; Lu, A. Down-regulation of Treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from Radix Glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules 2011, 16, 8343–8352. [Google Scholar] [CrossRef]
- Kasagi, S.; Kawano, S.; Kumagai, S. PD-1 and autoimmunity. Crit. Rev. Immunol. 2011, 31, 265–295. [Google Scholar]
- Chikuma, S.; Kanamori, M.; Mise-Omata, S.; Yoshimura, A. Suppressors of cytokine signaling: Potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci. 2017, 108, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tong, J.; Li, Z. Qiyusanlong decoction inhibits the level of PD-1/PD-L1 in mice bearing Lewis lung carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2016, 32, 770–774. [Google Scholar]
- Lv, J.; Jia, Y.; Li, J.; Kuai, W.; Li, Y.; Guo, F.; Xu, X.; Zhao, Z.; Lv, J.; Li, Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.-J.; Lu, Z.-Q.; Tang, L.-M.; Wu, Z.-S.; Wang, D.-W.; Zheng, J.-Y.; Qiu, Q.-M. Curcumin inhibits suppressive capacity of naturally occurring CD4+ CD25+ regulatory T cells in mice in vitro. Int. Immunopharmacol. 2012, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, A.; Sethi, G. Bioactive Natural Products in Cancer Prevention and Therapy: Progress and Promise. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–3. [Google Scholar]
- Wang, L.; Sun, J.; Gao, P.; Su, K.; Wu, H.; Li, J.; Lou, W. Wnt1-inducible signaling protein 1 regulates laryngeal squamous cell carcinoma glycolysis and chemoresistance via the YAP1/TEAD1/GLUT1 pathway. J. Cell. Physiol. 2019, 234, 15941–15950. [Google Scholar] [CrossRef]
- Zhang, P.; Lai, Z.-L.; Chen, H.-F.; Zhang, M.; Wang, A.; Jia, T.; Sun, W.-Q.; Zhu, X.-M.; Chen, X.-F.; Zhao, Z.; et al. Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J. Exp. Clin. Cancer Res. 2017, 36, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namkaew, J.; Jaroonwitchawan, T.; Rujanapun, N.; Saelee, J.; Noisa, P. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH-SY5Y human neuroblastoma cells. Vitr. Cell. Dev. Biol.-Anim. 2018, 54, 629–639. [Google Scholar] [CrossRef]
- Öztürk, Y.; Günaydın, C.; Yalçın, F.; Nazıroğlu, M.; Braidy, N. Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxidative Med. Cell. Longev. 2019, 2019, 4619865. [Google Scholar] [CrossRef]
- Shen, W.; Liang, B.; Yin, J.; Li, X.; Cheng, J. Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cell Line SKOV3/DDP to Cisplatin by Regulating Cell Cycle and Activating Apoptotic Pathways. Cell Biochem. Biophys. 2015, 72, 203–213. [Google Scholar] [CrossRef]
- Sivalingam, K.S.; Paramasivan, P.; Weng, C.F.; Viswanadha, V.p. Neferine Potentiates the Antitumor Effect of Cisplatin in Human Lung Adenocarcinoma Cells Via a Mitochondria-Mediated Apoptosis Pathway. J. Cell. Biochem. 2017, 118, 2865–2876. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.-L.; Liu, Y.-D.; Yang, L.-Y.; Jiang, Q.-K.; Zhu, X.-J.; Fan, H.-N.; Qian, Y. Cryptotanshinone sensitizes antitumor effect of paclitaxel on tongue squamous cell carcinoma growth by inhibiting the JAK/STAT3 signaling pathway. Biomed. Pharmacother. 2017, 95, 1388–1396. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, Q.; Wang, Y.; Fan, H.-w.; Li, Y. Synergistic anticancer effects of formononetin and temozolomide on glioma C6 cells. Biol. Pharm. Bull. 2018, 8, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.-S.; Wang, Y.-F.; Tzeng, Y.-M.; Chen, D.-R.; Liao, Y.-F.; Chiu, H.-Y.; Hsieh, W.-T. Aloe-Emodin Enhances Tamoxifen Cytotoxicity by Suppressing Ras/ERK and PI3K/mTOR in Breast Cancer Cells. Am. J. Chin. Med. 2017, 45, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.G.; Bort, A.; Mateos-Gómez, P.A.; Rodríguez-Henche, N.; Díaz-Laviada, I. Combination of the natural product capsaicin and docetaxel synergistically kills human prostate cancer cells through the metabolic regulator AMP-activated kinase. Cancer Cell Int. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, M.; Retnakumari, A.P.; Anwar, S.; Anto, N.P.; Mittal, R.; Shah, S.; Pillai, K.S.; Balachandran, V.S.; Peter, V.; Thomas, R.; et al. Heteronemin, a marine natural product, sensitizes acute myeloid leukemia cells towards cytarabine chemotherapy by regulating farnesylation of Ras. Oncotarget 2018, 9, 18115–18127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.-W.; Cheng, K.-J.; Mei, X.-L.; Qiu, J.-G.; Zhang, W.-J.; Xue, Y.-Q.; Qin, W.-M.; Yang, Y.; Zheng, D.-W.; Chen, Y.; et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget 2015, 6, 32790–32804. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, K.; Gu, C.; Yu, G.; Zhao, D.; Mai, W.; Zhong, Y.; Liu, S.; Nie, Y.; Yang, H. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol. Rep. 2018, 40, 1525–1532. [Google Scholar] [CrossRef] [Green Version]
- Desai, V.; Jain, A.; Shaghaghi, H.; Summer, R.; Lai, J.C.K.; Bhushan, A. Combination of biochanin A and temozolomide impairs tumor growth by modulating cell metabolism in glioblastoma multiforme. Anticancer Res. 2019, 39, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis-Stoman, D.; Du Preez, J.G.H.; van de Venter, M. Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 177–184. [Google Scholar]
- Lin, M.-T.; Lin, C.-L.; Lin, T.-Y.; Cheng, C.-W.; Yang, S.-F.; Lin, C.-L.; Wu, C.-C.; Hsieh, Y.-H.; Tsai, J.-P. Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 2016, 37, 6987–6996. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Ding, F.; Guo, S.; Ying, G.; Yan, Z. Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating O(6)-methylguanine-DNA methyltransferase (MGMT) expression. Am. J. Transl. Res. 2016, 8, 3299–3308. [Google Scholar]
- Neitzel, C.; Seiwert, N.; Göder, A.; Diehl, E.; Weber, C.; Nagel, G.; Stroh, S.; Rasenberger, B.; Christmann, M.; Fahrer, J. Lipoic Acid Synergizes with Antineoplastic Drugs in Colorectal Cancer by Targeting p53 for Proteasomal Degradation. Cells 2019, 8, 794. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhang, Y.; Li, H.; Zhou, Y.; Zhang, Q.; Chen, R.; Jin, T.; Hu, K.; Li, S.; Wang, Y.; et al. Vitamin D promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting LCN2-modulated NF-κB pathway activation through RPS3. Cell Death Dis. 2019, 10, 936. [Google Scholar] [CrossRef] [Green Version]
- Pazhang, Y.; Jaliani, H.Z.; Imani, M.; Dariushnejad, H. Synergism between NF-kappa B inhibitor, celastrol, and XIAP inhibitor, embelin, in an acute myeloid leukemia cell line, HL-60. J. Cancer Res. Ther. 2016, 12, 155. [Google Scholar]
- Li, X.; Zhu, F.; Jiang, J.; Sun, C.; Wang, X.; Shen, M.; Tian, R.; Shi, C.; Xu, M.; Peng, F.; et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 2015, 357, 219–230. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Lee, K.-T.; Park, H.-J.; Kim, T.J.; Choi, Y.S.; Shih, I.-M.; Choi, J.-H. Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway. Carcinogenesis 2012, 33, 2488–2498. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Xia, M.; Liu, W.; Li, L.; Yang, J.; Mei, Y.; Meng, Q.; Xie, Y. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Eur. J. Pharmacol. 2019, 852, 231–243. [Google Scholar] [CrossRef]
- Xia, G.; Wang, H.; Song, Z.; Meng, Q.; Huang, X.; Huang, X. Gambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2). J. Exp. Clin. Cancer Res. 2017, 36, 107. [Google Scholar] [CrossRef] [Green Version]
- Boueroy, P.; Hahnvajanawong, C.; Boonmars, T.; Saensa-ard, S.; Wattanawongdon, W.; Kongsanthia, C.; Salao, K.; Wongwajana, S.; Anantachoke, N.; Reutrakul, V. Synergistic Effect of Forbesione From Garcinia hanburyi in Combination with 5-Fluorouracil on Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2017, 18, 3343–3351. [Google Scholar] [CrossRef]
- Su, J.; Zhang, F.; Li, X.; Liu, Z. Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression. Biochem. Biophys. Res. Commun. 2019, 514, 510–517. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Sun, W.; Chen, R.; Wang, Z.; Cui, X.; Zhang, H.; Qian, S.; Zheng, Q.; Zhou, Y.; Wan, J.; et al. Pharmacokinetics in rat plasma and tissue distribution in mice of galangin determined by UHPLC–MS/MS. Acta Chromatogr. 2019, 31, 120–125. [Google Scholar] [CrossRef]
- Hung, J.-Y.; Yang, C.-J.; Tsai, Y.-M.; Huang, H.-W.; Huang, M.-S. Antiproliferative activity of aucubin is through cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Clin. Exp. Pharmacol. Physiol. 2008, 35, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-T.; Li, T.-Z.; Li, S.-M.; Wang, C.; Wang, H.; Luo, Y.-H.; Piao, X.-J.; Wang, J.-R.; Zhang, Y.; Zhang, T.; et al. Cytisine exerts anti-tumour effects on lung cancer cells by modulating reactive oxygen species-mediated signalling pathways. Artif. Cells Nanomed. Biotechnol. 2020, 48, 84–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Yang, X.; Pan, Y.; Qi, Q.; Shen, J.; Fang, H.; Ji, Z. L-securinine inhibits the proliferation of A549 lung cancer cells and promotes DKK1 promoter methylation. Oncol. Lett. 2017, 14, 4243–4248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Haque, E.; Hameed, R.; Maier, P.N.; Irfan, S.; Kamil, M.; Nazir, A.; Mir, S.S. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020, 256, 118000. [Google Scholar] [CrossRef] [PubMed]
- Srinual, S.; Chanvorachote, P.; Pongrakhananon, V. Suppression of cancer stem-like phenotypes in NCI-H460 lung cancer cells by vanillin through an Akt-dependent pathway. Int. J. Oncol. 2017, 50, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Hua, P.; Sun, M.; Zhang, G.; Zhang, Y.; Tian, X.; Li, X.; Cui, R.; Zhang, X. Cepharanthine induces apoptosis through reactive oxygen species and mitochondrial dysfunction in human non-small-cell lung cancer cells. Biochem. Biophys. Res. Commun. 2015, 460, 136–142. [Google Scholar] [CrossRef]
- Hutchinson, L. Breast cancer: Challenges, controversies, breakthroughs. Nat. Rev. Clin. Oncol. 2010, 7, 669–670. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Yue, G.G.-L.; Tsui, S.K.-W.; Pu, J.; Fung, K.-P.; Lau, C.B.-S. Elaborating the role of natural products on the regulation of autophagy and their potentials in breast cancer therapy. Curr. Cancer Drug Targets 2018, 18, 239–255. [Google Scholar] [CrossRef]
- Jin, Z.-Q.; Hao, J.; Yang, X.; He, J.-H.; Liang, J.; Yuan, J.-W.; Mao, Y.; Liu, D.; Cao, R.; Wu, X.-Z.; et al. Higenamine enhances the antitumor effects of cucurbitacin B in breast cancer by inhibiting the interaction of AKT and CDK2. Oncol. Rep. 2018, 40, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Rahman, H.; Rosli, R. In vivo anti-tumor effects of citral on 4T1 breast cancer cells via induction of apoptosis and downregulation of aldehyde dehydrogenase activity. Molecules 2019, 24, 3241. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Yang, D.; Guo, X.; Xu, Y.; Feng, B.; Kang, J. Euphol arrests breast cancer cells at the G1 phase through the modulation of cyclin D1, p21 and p27 expression. Mol. Med. Rep. 2013, 8, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.; Ghosh, P.; Kumavath, R. Strophanthidin Attenuates MAPK, PI3K/AKT/mTOR, and Wnt/β-Catenin Signaling Pathways in Human Cancers. Front. Oncol. 2020, 9, 1469. [Google Scholar] [CrossRef] [Green Version]
- Fatima, I.; El-Ayachi, I.; Taotao, L.; Lillo, M.A.; Krutilina, R.; Seagroves, T.N.; Radaszkiewicz, T.W.; Hutnan, M.; Bryja, V.; Krum, S.A.; et al. The natural compound Jatrophone interferes with Wnt/β-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS ONE 2017, 12, e0189864. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.-T.; Huang, H.-C.; Lin, J.-K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef]
- Dhandayuthapani, S.; Perez, H.D.; Paroulek, A.; Chinnakkannu, P.; Kandalam, U.; Jaffe, M.; Rathinavelu, A. Bromelain-Induced Apoptosis in GI-101A Breast Cancer Cells. J. Med. Food 2012, 15, 344–349. [Google Scholar] [CrossRef]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases in vitro. BMC Complement. Altern. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Stewart, B.; Wild, C.P. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Lee, C.S.; Jang, E.-R.; Kim, Y.J.; Myung, S.C.; Kim, W.; Lee, M.W. Diarylheptanoid hirsutenone enhances apoptotic effect of TRAIL on epithelial ovarian carcinoma cell lines via activation of death receptor and mitochondrial pathway. Investig. New Drugs 2012, 30, 548–557. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, Y.; Shi, C.; Song, X.; Chang, Y.; Ren, Y.; Shi, X. Berbamine suppresses cell proliferation and promotes apoptosis in ovarian cancer partially via the inhibition of Wnt/β-catenin signaling. Acta Biochim. Biophys. Sin. 2018, 50, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.G.; Li, L.Y.; Wei, Y.R.; Zhang, L.W. Screening, identification and activity evaluation of pancreatic lipase inhibition in Prunella vulgaris. China J. Chin. Mater. Med. 2018, 43, 4665–4671. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA 2017, 318, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ding, C.; Yang, Z.; Liu, T.; Zhang, X.; Zhao, C.; Wang, J. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J. Transl. Med. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, H.; Sawada, K.; Sato, A.; Nishi, K.; Sasaki, T.; Takahashi, T.; Ohori, H. Complete resection of liver metastases of colorectal cancer after high efficacy bevacizumab, S-1, and CPT-11 combination chemotherapy. Gan Kagaku Ryoho Cancer Chemother. 2015, 42, 101–104. [Google Scholar]
- Murthy, K.C.; Jayaprakasha, G.; Patil, B.S. Obacunone and obacunone glucoside inhibit human colon cancer (SW480) cells by the induction of apoptosis. Food Chem. Toxicol. 2011, 49, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Sithara, T.; Arun, K.; Syama, H.; Reshmitha, T.; Nisha, P. Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of warburg effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Ban, J.O.; Oh, J.H.; Hwang, B.Y.; Moon, D.C.; Jeong, H.-S.; Lee, S.; Kim, S.; Lee, H.; Kim, K.-B.; Han, S.B.; et al. Inflexinol inhibits colon cancer cell growth through inhibition of nuclear factor-κB activity via direct interaction with p50. Mol. Cancer Ther. 2009, 8, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Zhao, J.; Fan, D.; Wang, Z.; Zhao, T.; Li, Y.; Zhao, Y.; Adelson, D.; Hao, H. Alkaloids from nux vomica suppresses colon cancer cell growth through Wnt/β-catenin signaling pathway. Phytother. Res. 2019, 33, 1570–1578. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Zhang, J.; Lin, L.; Yao, Q.; Xiang, G. Bergenin suppresses the growth of colorectal cancer cells by inhibiting PI3K/AKT/mTOR signaling pathway. Trop. J. Pharm. Res. 2017, 16, 2307–2313. [Google Scholar] [CrossRef]
- Jin, Z.; Yan, W.; Jin, H.; Ge, C.; Xu, Y. Differential effect of psoralidin in enhancing apoptosis of colon cancer cells via nuclear factor-κB and B-cell lymphoma-2/B-cell lymphoma-2-associated X protein signaling pathways. Oncol. Lett. 2016, 11, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.-Y.; Chen, M.-H.; May, B.H.; Liao, X.-Z.; Liu, J.-H.; Tao, L.-T.; Man-yuen Sze, D.; Zhang, A.L.; Mo, S.-L. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine 2018, 51, 214–225. [Google Scholar] [CrossRef]
- Su, C.-M.; Weng, Y.-S.; Kuan, L.-Y.; Chen, J.-H.; Hsu, F.-T. Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 3527. [Google Scholar] [CrossRef]
- Mi, C.; Ma, J.; Wang, K.S.; Zuo, H.X.; Wang, Z.; Li, M.Y.; Piao, L.X.; Xu, G.H.; Li, X.; Quan, Z.S.; et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 2017, 203, 27–38. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Deng, H.; Liang, L.; Peng, J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Med. 2014, 33, 1613–1620. [Google Scholar] [CrossRef] [Green Version]
- McNeill, K.A. Epidemiology of brain tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.-N.; Yu, M.-Y.; Kong, L.-M.; Wang, W.-H.; Yang, Y.-F.; Liu, J.-Q.; Qiu, M.-H.; Li, Y. Biflavone ginkgetin, a novel Wnt inhibitor, suppresses the growth of medulloblastoma. Nat. Prod. Bioprospect. 2015, 5, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Qu, D.; Wang, H.; Zhang, S.; Jia, C.; Shi, Z.; Wang, Z.; Zhang, J.; Ma, J. Toosendanin exerts an anti-cancer effect in glioblastoma by inducing estrogen receptor β- and p53-mediated apoptosis. Int. J. Mol. Sci. 2016, 17, 1928. [Google Scholar] [CrossRef] [Green Version]
- Noman, L.; Oke-Altuntas, F.; Zellagui, A.; Sahin Yaglioglu, A.; Demirtas, I.; Cardoso, S.M.; Akkal, S.; Gherraf, N.; Rhouati, S. A novel benzimidazole and other constituents with antiproliferative and antioxidant properties from Thymelaea microphylla Coss. et Dur. Nat. Prod. Res. 2017, 31, 2032–2041. [Google Scholar] [CrossRef]
- Schötterl, S.; Hübner, M.; Armento, A.; Veninga, V.; Wirsik, N.M.; Bernatz, S.; Lentzen, H.; Mittelbronn, M.; Naumann, U. Viscumins functionally modulate cell motility-associated gene expression. Int. J. Oncol. 2017, 50, 684–696. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, Y.; Li, S.; Gan, R.-Y.; Li, H.-B. Natural Products for Prevention and Treatment of Chemical-Induced Liver Injuries. Compr. Rev. Food Sci. Food Saf. 2018, 17, 472–495. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.-B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Wang, J.-F.; Li, H.; Long, T.-T.; Li, Z.; Wang, Y.-M.; Dong, P.; Xue, C.-H. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuria graeffei. J. Sci. Food Agric. 2012, 92, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-M.; Chai, E.-Q.; Cai, H.-Y.; Miao, G.-Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Liu, B.-B.; Qian, X.-D.; Li, L.-Q.; Cao, H.-B.; Guo, Q.-S.; Zhou, G.-F. Crocin induces autophagic apoptosis in hepatocellular carcinoma by inhibiting Akt/mTOR activity. OncoTargets Ther. 2018, 11, 2017–2028. [Google Scholar] [CrossRef]
- Hui, F.; Qin, X.; Zhang, Q.; Li, R.; Liu, M.; Ren, T.; Zhao, M.; Zhao, Q. Alpinia oxyphylla oil induces apoptosis of hepatocellular carcinoma cells via PI3K/Akt pathway in vitro and in vivo. Biomed. Pharmacother. 2019, 109, 2365–2374. [Google Scholar] [CrossRef]
- García-Fernández, L.F.; Losada, A.; Alcaide, V.; Álvarez, A.M.; Cuadrado, A.; González, L.; Nakayama, K.; Nakayama, K.I.; Fernández-Sousa, J.M.; Muñoz, A.; et al. Aplidin™ induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C δ. Oncogene 2002, 21, 7533–7544. [Google Scholar] [CrossRef] [Green Version]
- Ming, Y.; Zheng, Z.; Chen, L.; Zheng, G.; Liu, S.; Yu, Y.; Tong, Q. Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell Biol. Int. 2013, 37, 1046–1054. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Yang, I.-H.; Shin, J.-A.; Kim, L.-H.; Kwon, K.H.; Cho, S.-D. The caspase 3-dependent apoptotic effect of pycnogenol in human oral squamous cell carcinoma HSC-3 cells. J. Clin. Biochem. Nutr. 2016, 58, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.-H.; Kim, I.-R.; Kim, H.-J.; Park, B.-S.; Yu, S.-B. α-Mangostin Induces Apoptosis and Cell Cycle Arrest in Oral Squamous Cell Carcinoma Cell. Evid.-Based Complement. Altern. Med. 2016, 2016, 9060649. [Google Scholar] [CrossRef]
- De La Chapa, J.J.; Singha, P.K.; Lee, D.R.; Gonzales, C.B. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J. Oral Pathol. Med. 2018, 47, 674–682. [Google Scholar] [CrossRef]
- Chattopadhyay, I. Role of Nutrigenetics and Nutrigenomics in Cancer Chemoprevention. In Pharmacotherapeutic Botanicals for Cancer Chemoprevention; Springer: Singapore, 2020; pp. 167–188. [Google Scholar]
- Katiyar, S.K. Emerging phytochemicals for the prevention and treatment of head and neck cancer. Molecules 2016, 21, 1610. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, C.F.; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11, 1483. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.; Gandhi, J.; Joshi, G.; Smith, N.L.; Khan, S.A. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 351–361. [Google Scholar] [CrossRef]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.T.; Gupta, S. Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.-C.; Sun, J.-M.; Shen, J.-G.; Jin, J.-Z.; Liu, F.; Xu, X.-L.; Chen, L.; Liu, L.-T.; Lv, J.-J. Afzelin exhibits anti-cancer activity against androgen-sensitive LNCaP and androgen-independent PC-3 prostate cancer cells through the inhibition of LIM domain kinase 1. Oncol. Lett. 2015, 10, 2359–2365. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Syed, D.N.; Khan, M.I.; Adhami, V.M.; Mirza, B.; Mukhtar, H. The pentacyclic triterpenoid, plectranthoic acid, a novel activator of AMPK induces apoptotic death in prostate cancer cells. Oncotarget 2016, 7, 3819–3831. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Jiang, X.; Yin, G.; He, L.; Liu, J.; Long, Z.; Jiang, Z.; Yao, K. Anacardic acid induces cell apoptosis of prostatic cancer through autophagy by ER stress/DAPK3/Akt signaling pathway. Oncol. Rep. 2017, 38, 1373–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.-H.; Ko, H.; Jeon, H.; Sung, G.-J.; Park, S.-Y.; Jun, W.J.; Lee, Y.-H.; Lee, J.; Lee, S.-w.; Yoon, H.-G.; et al. Delphinidin induces apoptosis via cleaved HDAC3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget 2016, 7, 56767–56780. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Peng, S.; He, Y.; Qin, M.; Cong, X.; Xing, Y.; Liu, M.; Yi, Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget 2015, 6, 15348–15361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lall, R.K.; Syed, D.N.; Khan, M.I.; Adhami, V.M.; Gong, Y.; Lucey, J.A.; Mukhtar, H. Dietary flavonoid fisetin increases abundance of high-molecular-mass hyaluronan conferring resistance to prostate oncogenesis. Carcinogenesis 2016, 37, 918–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adaramoye, O.; Erguen, B.; Nitzsche, B.; Höpfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem.-Biol. Interact. 2017, 274, 100–106. [Google Scholar] [CrossRef]
- Zeng, S.; Zhu, B.; Zeng, J.; Wu, W.; Jiang, C. Zeylenone represses the progress of human prostate cancer by downregulating the Wnt/β-catenin pathway. Mol. Med. Rep. 2018, 18, 5572–5578. [Google Scholar] [CrossRef] [Green Version]
- Núñez Selles, A.J.; Daglia, M.; Rastrelli, L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. BioFactors 2016, 42, 475–491. [Google Scholar] [CrossRef]
- Elkady, A.I. Anethole inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and apoptosis. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents 2018, 18, 216–236. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.-Y.; Lei, Z.-M.; Wan, L.-J.; Zhu, X.-W.; Ye, F.; Tong, Y.-Y. Anti-proliferative effects of paeonol on human prostate cancer cell lines DU145 and PC-3. J. Physiol. Biochem. 2017, 73, 157–165. [Google Scholar] [CrossRef]
- Kanwal, N.; Rasul, A.; Hussain, G.; Anwar, H.; Shah, M.A.; Sarfraz, I.; Riaz, A.; Batool, R.; Shahbaz, M.; Hussain, A.; et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem. Toxicol. 2020, 143, 111570. [Google Scholar] [CrossRef]
- Ramamoorthy, M.D.; Kumar, A.; Ayyavu, M.; Dhiraviam, K.N.; Ayyau, M. Reserpine induces apoptosis and cell cycle arrest in hormone independent prostate cancer cells through mitochondrial membrane potential failure. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents 2018, 18, 1313–1322. [Google Scholar] [CrossRef]
- Royston, K.J.; Tollefsbol, T.O. The epigenetic impact of cruciferous vegetables on cancer prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. JNCI J. Natl. Cancer Inst. 1981, 66, 66–1192. [Google Scholar] [CrossRef]
- Kang, B.; Park, H.; Kim, B. Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 2302. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Giaisi, M.; Köhler, R.; Chen, W.-M.; Krammer, P.H.; Li-Weber, M. Rocaglamide breaks TRAIL-resistance in human multiple myeloma and acute T-cell leukemia in vivo in a mouse xenogtraft model. Cancer Lett. 2017, 389, 70–77. [Google Scholar] [CrossRef]
- Ishii, N.; Araki, K.; Yokobori, T.; Hagiwara, K.; Gantumur, D.; Yamanaka, T.; Handa, T.; Tsukagoshi, M.; Igarashi, T.; Watanabe, A.; et al. Conophylline suppresses pancreatic cancer desmoplasia and cancer-promoting cytokines produced by cancer-associated fibroblasts. Cancer Sci. 2019, 110, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-H.; Yi, E.H.; Jee, J.-G.; Jeong, A.J.; Sandoval, C.; Park, I.-C.; Baeg, G.H.; Ye, S.-K. Tubulosine selectively inhibits JAK3 signalling by binding to the ATP-binding site of the kinase of JAK3. J. Cell. Mol. Med. 2020, 24, 7427–7438. [Google Scholar] [CrossRef]
- Karami, A.; Hamzeloo-Moghadam, M.; Yami, A.; Barzegar, M.; Mashati, P.; Gharehbaghian, A. Antiproliferative Effect of Gaillardin from Inula oculus-christi in Human Leukemic Cells. Nutr. Cancer 2020, 72, 1043–1056. [Google Scholar] [CrossRef]
- Trivedi, R.; Maurya, R.P.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef] [Green Version]
- Uchihara, Y.; Tago, K.; Funakoshi-Tago, M. The mechanisms of taxodione-induced apoptosis in BCR-ABL-positive leukemia cells. Nihon Yakurigaku Zasshi Folia Pharmacol. Jpn. 2019, 153, 147–154. [Google Scholar] [CrossRef]
- Wu, C.; Li, M.; Meng, H.; Liu, Y.; Niu, W.; Zhou, Y.; Zhao, R.; Duan, Y.; Zeng, Z.; Li, X.; et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci. China Life Sci. 2019, 62, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Shivamadhu, M.C.; Srinivas, B.K.; Jayarama, S.; Chandrashekaraiah, S.A. Anti-cancer and anti-angiogenic effects of partially purified lectin from Praecitrullus fistulosus fruit on in vitro and in vivo model. Biomed. Pharmacother. 2017, 96, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Park, J.Y.; Yu, J.S.; Lee, S.O.; Ryu, J.-Y.; Choi, S.-Z.; Kang, K.S.; Yamabe, N.; Kim, K.H. Odisolane, a Novel Oxolane Derivative, and Antiangiogenic Constituents from the Fruits of Mulberry (Morus alba L.). J. Agric. Food Chem. 2016, 64, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Yap, V.A.; Loong, B.-J.; Ting, K.-N.; Loh, S.H.-S.; Yong, K.-T.; Low, Y.-Y.; Kam, T.-S.; Lim, K.-H. Hispidacine, an unusual 8,4′-oxyneolignan-alkaloid with vasorelaxant activity, and hispiloscine, an antiproliferative phenanthroindolizidine alkaloid, from Ficus hispida Linn. Phytochemistry 2015, 109, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Kim, J.H.; Lee, Y.; Yang, H.; Heo, Y.-S.; Bode, A.M.; Lee, K.W.; Dong, Z. Bakuchiol suppresses proliferation of skin cancer cells by directly targeting Hck, Blk, and p38 MAP kinase. Oncotarget 2016, 7, 14616–14627. [Google Scholar] [CrossRef] [Green Version]
- Pal, H.C.; Katiyar, S.K. Cryptolepine, a plant alkaloid, inhibits the growth of non-melanoma skin cancer cells through inhibition of topoisomerase and induction of DNA damage. Molecules 2016, 21, 1758. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, Y.; Yang, R.; Zhou, T.; Ke, W.; Si, Y.; Yang, S.; Zhang, T.; Liu, X.; Zhang, L.; et al. Cucurbitacin B inhibits gastric cancer progression by suppressing STAT3 activity. Arch. Biochem. Biophys. 2020, 684, 108314. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A potential drug for cancer treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Xu, C.; Cao, X.; Wang, W. Isovitexin Suppresses Cancer Stemness Property And Induces Apoptosis Of Osteosarcoma Cells by Disruption of The DNMT1/miR-34a/Bcl-2 Axis. Cancer Manag. Res. 2019, 11, 8923. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.H. Melatonin and cancer hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Zu, X.; Liu, F.; Wang, T.; Wang, X.; Chen, H.; Liu, K.; Wang, P.; Liu, F.; Zheng, Y.; et al. Purpurogallin is a novel mitogen-activated protein kinase kinase 1/2 inhibitor that suppresses esophageal squamous cell carcinoma growth in vitro and in vivo. Mol. Carcinog. 2019, 58, 1248–1259. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, T.; Yang, N.; Li, Z.; Liu, Y. Anticancer effects of Mahanimbine alkaloid on the human bladder cancer cells are due to the induction of G0/G1 cell cycle arrest, apoptosis and autophagy. J. BUON 2020, 25, 1166–1171. [Google Scholar]
- Tu, S.; Zhang, X.L.; Wan, H.F.; Xia, Y.Q.; Liu, Z.Q.; Yang, X.H.; Wan, F.S. Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncol. Lett. 2018, 15, 5473–5480. [Google Scholar] [CrossRef]
- Rath, B.; Hochmair, M.; Plangger, A.; Hamilton, G. Anticancer activity of fascaplysin against lung cancer cell and small cell lung cancer circulating tumor cell lines. Mar. Drugs 2018, 16, 383. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.-J.; Gao, W.-N.; Wu, Q.-B.; Yao, X.-J.; Jiang, Z.-B.; Wang, Y.-W.; Wang, W.-J.; Li, W.; Hussain, S.; Liu, L.; et al. Chelidonine selectively inhibits the growth of gefitinib-resistant non-small cell lung cancer cells through the EGFR-AMPK pathway. Pharmacol. Res. 2020, 159, 104934. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, E.B.; Lee, J.; Jung, O.; Ryu, B.J.; Kim, S.H.; Cho, J.Y.; Ryou, C.; Lee, S.Y. Emetine inhibits migration and invasion of human non-small-cell lung cancer cells via regulation of ERK and p38 signaling pathways. Chem.-Biol. Interact. 2015, 242, 25–33. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Zhai, D.-D.; Supaibulwatana, K.; Zhong, J.-J. Inhibition of tumor cell proliferation and induction of apoptosis in human lung carcinoma 95-D cells by a new sesquiterpene from hairy root cultures of Artemisia annua. Phytomedicine 2010, 17, 856–861. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Guo, Y.; Li, G.; Xie, Q.; Zhu, B.; Gao, J.; Chen, Z. Artemisinin Inhibits Tumor Lymphangiogenesis by Suppression of Vascular Endothelial Growth Factor C. Pharmacology 2008, 82, 148–155. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Tao, C.; Xiao, T.; Li, X.; Zeng, Q.; Ma, M.; Wu, Z. Artemether attenuates the progression of non-small cell lung cancer by inducing apoptosis, cell cycle arrest and promoting cellular senescence. Biol. Pharm. Bull. 2019, 42, 1720–1725. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Noratto, G.D.; Li, X.; Angel-Morales, G.; Bertoldi, M.C.; Safe, S. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: Role of Sp transcription factors and microRNA-27a: ZBTB10. Mol. Carcinog. 2012, 52, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, T.-I.; Wang, M.-C.; Chen, S.-Y.; Huang, S.-T.; Yeh, Y.-M.; Su, W.-C.; Chang, W.-C.; Hung, J.-J. Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth. Mol. Pharmacol. 2012, 82, 1115–1128. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, M.; Jiang, Y.; Wu, H.; Lu, G.; Shi, W.; Cong, D.; Song, S.; Liu, K.; Wang, H. Gambogic Acid Induces Apoptosis of Non-Small Cell Lung Cancer (NSCLC) Cells by Suppressing Notch Signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7146–7151. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Zhou, S.; Zhu, L.; Ming, J.; Zeng, F.; Xu, R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- You, C.; Sun, Y.; Zhang, S.; Tang, G.; Zhang, N.; Li, C.; Tian, X.; Ma, S.; Luo, Y.; Sun, W.; et al. Trichosanthin enhances sensitivity of non-small cell lung cancer (NSCLC) TRAIL-resistance cells. Int. J. Biol. Sci. 2018, 14, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.S.; Zhou, H.; Yang, L.; Wang, L.; Jiang, Z.S.; Sun, H.; Wang, S.M. Ursolic Acid Attenuates TGF-β1-Induced Epithelial-Mesenchymal Transition in NSCLC by Targeting Integrin αVβ5/MMPs Signaling. Oncol. Res. 2019, 27, 593–600. [Google Scholar] [CrossRef]
- Song, Y.; Kong, L.; Sun, B.; Gao, L.; Chu, P.; Ahsan, A.; Qaed, E.; Lin, Y.; Peng, J.; Ma, X.; et al. Induction of autophagy by an oleanolic acid derivative, SZC017, promotes ROS-dependent apoptosis through Akt and JAK2/STAT3 signaling pathway in human lung cancer cells. Cell Biol. Int. 2017, 41, 1367–1378. [Google Scholar] [CrossRef]
- Wang, C.; Cui, C. Inhibition of lung cancer proliferation by wogonin is associated with the activation of apoptosis and generation of reactive oxygen species. Balk. Med. J. 2020, 37, 29–33. [Google Scholar] [CrossRef]
- Jiang, Z.-Q.; Li, M.-H.; Qin, Y.-M.; Jiang, H.-Y.; Zhang, X.; Wu, M.-H. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Yang, Y.; Yang, Y.; Liu, X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomed. Pharmacother. 2018, 108, 130–136. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Zheng, F.; Chen, Y.; Hann, S.S. Emodin increases expression of insulin-like growth factor binding protein 1 through activation of MEK/ERK/AMPKα and interaction of PPARγ and Sp1 in lung cancer. Cell. Physiol. Biochem. 2017, 41, 339–357. [Google Scholar] [CrossRef]
- Yao, C.-C.; Tu, Y.-R.; Jiang, J.; Ye, S.-F.; Du, H.-X.; Zhang, Y. β-elemene reverses the drug resistance of lung cancer A549/DDP cells via the mitochondrial apoptosis pathway. Oncol. Rep. 2014, 31, 2131–2138. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Luan, J.; Duan, H.; Zhang, F.; Yagasaki, K.; Zhang, G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology 2010, 62, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.-C.; Yang, M.-D.; Hsiao, Y.-T.; Lin, T.-S.; Ma, Y.-S.; Peng, S.-F.; Hsia, T.-C.; Cheng, Y.-D.; Kuo, C.-L.; Chung, J.-G. Bufalin inhibits gefitinib resistant NCI-H460 human lung cancer cell migration and invasion in vitro. J. Ethnopharmacol. 2016, 194, 1043–1050. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, M.; Jiang, Z.; Zhao, F.; Xi, B.; Zhang, X.; Fu, H.; Zhou, K. Platycodin-D Induced Autophagy in Non-Small Cell Lung Cancer Cells via PI3K/Akt/mTOR and MAPK Signaling Pathways. J. Cancer 2015, 6, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.; Jiang, J.-Y.; Liu, S.-D.; Fu, K.; Liu, H.-Y. Tanshinone IIA induces cytochrome c-mediated caspase cascade apoptosis in A549 human lung cancer cells via the JNK pathway. Int. J. Oncol. 2014, 45, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Mou, H.; Zheng, Y.; Zhao, P.; Bao, H.; Fang, W.; Xu, N. Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria-and Fas/FasL-mediated pathways. Toxicol. Vitr. 2011, 25, 1027–1032. [Google Scholar] [CrossRef]
- Piantino, C.B.; Salvadori, F.A.; Ayres, P.P.; Kato, R.B.; Srougi, V.; Leite, K.R.; Srougi, M. An evaluation of the anti-neoplastic activity of curcumin in prostate cancer cell lines. Int. Braz. J. Urol. 2009, 35, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Song, X.; Shang, M.; Zou, W.; Zhang, M.; Wei, H.; Shao, H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019, 15, 1243–1253. [Google Scholar] [CrossRef]
- Wu, C.; Zhuang, Y.; Jiang, S.; Tian, F.; Teng, Y.; Chen, X.; Zheng, P.; Liu, S.; Zhou, J.; Wu, J.; et al. Cinnamaldehyde induces apoptosis and reverses epithelial-mesenchymal transition through inhibition of Wnt/β-catenin pathway in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2017, 84, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, W.; Wu, J.; Li, M.; Wang, J.; Wu, J.; Luo, C. A polysaccharide fraction of adlay seed (Coix lachryma-jobi L.) induces apoptosis in human non-small cell lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2013, 430, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jiang, R.; Cheng, Z.; Lu, Y.; Gu, L.; Li, H.; Li, L.; Gao, Q.; Chen, M.; Zhang, X. Ophiopogonin-B suppresses epithelial-mesenchymal transition in human lung adenocarcinoma cells via the Linc00668/miR-432-5p/EMT axis. J. Cancer 2019, 10, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-R.; Zheng, J.; Sun, Q.; Wei, K.; Hu, Y. Radix Tetrastigma hemsleyani flavone inhibits proliferation, migration, and invasion of human lung carcinoma A549 cells. OncoTargets Ther. 2016, 9, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, P.-Y.; Huang, G.-J.; Pan, C.-H.; Chien, Y.-C.; Chen, Y.-Y.; Wu, C.-H.; Sheu, M.-J.; Cheng, H.-C. Trilinolein inhibits proliferation of human non-small cell lung carcinoma A549 through the modulation of pi3k/akt pathway. Am. J. Chin. Med. 2011, 39, 803–815. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Lu, M.-K.; Leng, P.-J.; Tsao, S.-M.; Wu, Y.-C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, srep44990. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Zhang, X.; Li, C.; Guan, H. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm. J. 2017, 25, 638–643. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Chen, Y.-G.; Shi, R.-Z.; He, K.; Wang, J.-F.; Shao, J.-H.; Wan, J.-B.; Gao, J.-L. 20(S)-Protopanaxdiol Suppresses the Abnormal Granule-Monocyte Differentiation of Hematopoietic Stem Cells in 4T1 Breast Cancer-Bearing Mouse. Evid.-Based Complement. Altern. Med. 2020, 2020, 8747023. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Jedinak, A.; Sliva, D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem. Biophys. Res. Commun. 2011, 415, 325–329. [Google Scholar] [CrossRef]
- Xiaoping, C.; Yan, C.; Shuibing, L.; Youguo, C.; Jianyun, L.; Lanping, L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.; Chen, G.; Hu, Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Yang, R.; Hanwell, H.; Zhang, J.; Tsao, R.; Meckling, K.A. Antiproliferative Activity of Pomiferin in Normal (MCF-10A) and Transformed (MCF-7) Breast Epithelial Cells. J. Agric. Food Chem. 2011, 59, 13328–13336. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, S.; Li, J.; Jiang, C.; Ye, M.; Hu, H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis 2011, 16, 394–403. [Google Scholar] [CrossRef]
- Yue, G.G.-L.; Xie, S.; Lee, J.K.-M.; Kwok, H.-F.; Gao, S.; Nian, Y.; Wu, X.-X.; Wong, C.-K.; Qiu, M.-H.; Lau, C.B.-S. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci. Rep. 2016, 6, 35263. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C 2019, 98, 685–695. [Google Scholar] [CrossRef]
- Cao, C.; Huang, W.; Zhang, N.; Wu, F.; Xu, T.; Pan, X.; Peng, C.; Han, B. Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif. 2018, 51, e12518. [Google Scholar] [CrossRef]
- Kong, Y.; Li, F.; Nian, Y.; Zhou, Z.; Yang, R.; Qiu, M.-H.; Chen, C. KHF16 is a Leading Structure from Cimicifuga foetida that Suppresses Breast Cancer Partially by Inhibiting the NF-κB Signaling Pathway. Theranostics 2016, 6, 875–886. [Google Scholar] [CrossRef] [Green Version]
- Hien, T.T.; Kim, H.G.; Han, E.H.; Kang, K.W.; Jeong, H.G. Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-κB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol. Nutr. Food Res. 2010, 54, 918–928. [Google Scholar] [CrossRef]
- Moon, A.; Kim, E.-S.; Jeong, C.-S. Genipin, a constituent of Gardenia jasminoides Ellis, induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol. Rep. 2012, 27, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Wang, X.-F.; Zhou, Q.-M.; Zhang, T.-L.; Lu, Y.-Y.; Zhang, H.; Su, S.-B. Evodiamine induces apoptosis and inhibits metastasis in MDA-MB-231 human breast cancer cells in vitro and in vivo. Oncol. Rep. 2013, 30, 685–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Chen, X.; Li, T.; Xu, J.; Ma, Y. A myrsinol diterpene isolated from a traditional herbal medicine, LANGDU reverses multidrug resistance in breast cancer cells. J. Ethnopharmacol. 2016, 194, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mohsenikia, M.; Alizadeh, A.M.; Khodayari, S.; Khodayari, H.; Kouhpayeh, S.A.; Karimi, A.; Zamani, M.; Azizian, S.; Mohagheghi, M.A. The protective and therapeutic effects of alpha-solanine on mice breast cancer. Eur. J. Pharmacol. 2013, 718, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.-Y.; Ren, Y.-D.; Du, S.-Y.; Zhang, M.; Wang, Y.-S.; Fang, L.; Zhang, H. Melosuavine I, an apoptosis-inducing bisindole alkaloid from Melodinus suaveolens. Fitoterapia 2019, 133, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, Y.; Shi, K.; Wang, H.; Lu, J.; Li, Y.; Ma, C. Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis. J. Biomed. Res. 2015, 29, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Liu, G.; Liang, M.; Xu, Y. Ophiopogonin D inhibits cell proliferation and induces apoptosis of human laryngocarcinoma through downregulation of cyclin B1 and MMP-9 and upregulation of p38-MAPK signaling. Oncol. Lett. 2019, 17, 1877–1882. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Chen, X.; Guo, B.; Huang, W.; Shen, T.; Sun, X.; Xiao, P.; Zhou, Q. Induction of apoptosis by Icariside II through extrinsic and intrinsic signaling pathways in human breast cancer MCF7 cells. Biosci. Biotechnol. Biochem. 2012, 76, 1322–1328. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Z.; Wang, X.; Zhang, X.; Wang, M.; Wang, Y.; Mei, Q.; Wang, Z. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp. Mol. Med. 2012, 44, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-H.; Nao, J.-F.; Zhang, M.; He, P. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumor Biol. 2014, 35, 11985–11994. [Google Scholar] [CrossRef]
- Woo, J.-H.; Ahn, J.-H.; Jang, D.S.; Lee, K.-T.; Choi, J.-H. Effect of kumatakenin isolated from cloves on the apoptosis of cancer cells and the alternative activation of tumor-associated macrophages. J. Agric. Food Chem. 2017, 65, 7893–7899. [Google Scholar] [CrossRef]
- Hua, F.; Li, C.-H.; Chen, X.-G.; Liu, X.-P. Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the Raf/MEK/ERK cascade. Int. J. Mol. Med. 2018, 41, 3485–3492. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-L.; Su, J.-H.; Yeh, Y.-T.; Lee, Y.-C.; Chen, H.-M.; Wu, Y.-C.; Yuan, S.-S.F. Protoapigenone, a novel flavonoid, inhibits ovarian cancer cell growth in vitro and in vivo. Cancer Lett. 2008, 267, 85–95. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Chen, M.; Ouyang, Y.; Li, R.; Zhang, X.; Gao, X.; Lin, S.; Wang, X. Icaritin induces ovarian cancer cell apoptosis through activation of p53 and inhibition of Akt/mTOR pathway. Life Sci. 2018, 202, 188–194. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lim, W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, X.-C.; Cao, J.-G.; Liu, F.; Quan, M.-F.; Sheng, X.-F.; Ren, K.-Q. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol. Lett. 2013, 5, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Y.; Zhang, F.; Zhang, Y.-S.; Thakur, K.; Zhang, J.-G.; Liu, Y.; Kan, H.; Wei, Z.-J. Mechanism of juglone-induced cell cycle arrest and apoptosis in Ishikawa Human endometrial cancer cells. J. Agric. Food Chem. 2019, 67, 7378–7389. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Chen, A.Y.; Ye, X.; Luo, H.; Rankin, G.O.; Chen, Y.C. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013, 14, 6012–6025. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Feng, J.; Wang, R.; Zhang, H.; Liu, J. Effects of flavonoids from Potamogeton crispus L. on proliferation, migration, and invasion of human ovarian cancer cells. PLoS ONE 2015, 10, e0130685. [Google Scholar] [CrossRef] [Green Version]
- Greenshields, A.L.; Shepherd, T.G.; Hoskin, D.W. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 2017, 56, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, L.A.; Meng, Y.G.; You, Y.Q.; Fu, X.Y.; Song, L. Arctigenin promotes apoptosis in ovarian cancer cells via the iNOS/NO/STAT3/survivin signalling. Basic Clin. Pharmacol. Toxicol. 2014, 115, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhang, Y.-Y.; Sun, Y.-S.; Ma, R.-H.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Asparanin A from Asparagus officinalis L. Induces G0/G1 Cell Cycle Arrest and Apoptosis in Human Endometrial Carcinoma Ishikawa Cells via Mitochondrial and PI3K/AKT Signaling Pathways. J. Agric. Food Chem. 2020, 68, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, J.; Ren, B.; Zhang, L.; Owusu, L.; Liu, L.; Zhang, J.; Tang, Y.; Li, W. Anti-tumor and chemosensitization effects of Cryptotanshinone extracted from Salvia miltiorrhiza Bge. on ovarian cancer cells in vitro. J. Ethnopharmacol. 2017, 205, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, C.; Wang, X.; Zhai, C.; Yi, Z.; Wang, L.; Liu, B.; Du, B.; Wu, H.; Guo, X.; et al. Dauricine induces apoptosis, inhibits proliferation and invasion through inhibiting NF-κB signaling pathway in colon cancer cells. J. Cell. Physiol. 2010, 225, 266–275. [Google Scholar] [CrossRef]
- Xia, S.; Miao, Y.; Liu, S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2018, 503, 2363–2369. [Google Scholar] [CrossRef]
- De Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef]
- Ray, P.; Guha, D.; Chakraborty, J.; Banerjee, S.; Adhikary, A.; Chakraborty, S.; Das, T.; Sa, G. Crocetin exploits p53-induced death domain (PIDD) and FAS-associated death domain (FADD) proteins to induce apoptosis in colorectal cancer. Sci. Rep. 2016, 6, 32979. [Google Scholar] [CrossRef]
- Lee, S.K.; Nam, K.-A.; Heo, Y.-H. Cytotoxic activity and G2/M cell cycle arrest mediated by antofine, a phenanthroindolizidine alkaloid isolated from Cynanchum paniculatum. Planta Med. 2003, 69, 21–25. [Google Scholar] [CrossRef]
- Sithara, T.; Dhanya, B.P.; Arun, K.B.; Sini, S.; Dan, M.; Kokkuvayil Vasu, R.; Nisha, P. Zerumbone, a cyclic sesquiterpene from Zingiber zerumbet induces apoptosis, cell cycle arrest, and antimigratory effects in SW480 colorectal cancer cells. J. Agric. Food Chem. 2018, 66, 602–612. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, Y.-J.; Liu, D.-Y.; Shen, R.-R. Pancratistatin Inhibits the Growth of Colorectal Cancer Cells by Inducing Apoptosis, Autophagy, and G2/M Cell Cycle Arrest. Med. Sci. Monit. 2019, 25, 6015–6022. [Google Scholar] [CrossRef]
- Dong, G.-Z.; Shim, A.-R.; Hyeon, J.S.; Lee, H.J.; Ryu, J.-H. Inhibition of Wnt/β-catenin pathway by dehydrocostus lactone and costunolide in colon cancer cells. Phytother. Res. 2015, 29, 680–686. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Y.; Yang, A.; Fu, X.; Mao, M.; Liu, Z. Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharmacother. 2017, 95, 68–76. [Google Scholar] [CrossRef]
- Go, H.; Hwang, H.-J.; Nam, T.-J. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. Toxicol. Vitr. 2010, 24, 1546–1553. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kee, J.-Y.; Hong, S.-H. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front. Pharmacol. 2018, 9, 68. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Patil, B.S. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur. J. Pharmacol. 2012, 688, 14–21. [Google Scholar] [CrossRef]
- Han, M.H.; Kim, G.-Y.; Yoo, Y.H.; Choi, Y.H. Sanguinarine induces apoptosis in human colorectal cancer HCT-116 cells through ROS-mediated Egr-1 activation and mitochondrial dysfunction. Toxicol. Lett. 2013, 220, 157–166. [Google Scholar] [CrossRef]
- Sichaem, J.; Surapinit, S.; Siripong, P.; Khumkratok, S.; Jong-aramruang, J.; Tip-pyang, S. Two new cytotoxic isomeric indole alkaloids from the roots of Nauclea orientalis. Fitoterapia 2010, 81, 830–833. [Google Scholar] [CrossRef]
- Ji, S.; Tang, S.; Li, K.; Li, Z.; Liang, W.; Qiao, X.; Wang, Q.; Yu, S.; Ye, M. Licoricidin inhibits the growth of SW480 human colorectal adenocarcinoma cells in vitro and in vivo by inducing cycle arrest, apoptosis and autophagy. Toxicol. Appl. Pharmacol. 2017, 326, 25–33. [Google Scholar] [CrossRef]
- Jayameena, P.; Sivakumarik, A.K.; Rajesh, S. Rutin: A potential anticancer drug against human colon cancer (Hct116) cells. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 1731–1745. [Google Scholar]
- Dou, J.; Wang, Z.; Ma, L.; Peng, B.; Mao, K.; Li, C.; Su, M.; Zhou, C.; Peng, G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018, 9, 20089–20102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabana, Y.M.; Hassan, L.E.A.; Ahamed, M.B.K.; Dahham, S.S.; Iqbal, M.A.; Saeed, M.A.A.; Khan, S.S.; Sandai, D.; Majid, A.S.A.; Oon, C.E.; et al. Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc. Res. 2016, 107, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Won, S.-J.; Chao, C.-L.; Wu, F.-L.; Liu, H.-S.; Ling, P.; Lin, C.-N.; Su, C.-L. Morusin induces apoptosis and suppresses NF-κB activity in human colorectal cancer HT-29 cells. Biochem. Biophys. Res. Commun. 2008, 372, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.M.; Li, W.; Gray, Z.; Matter, M.S.; Colburn, N.H.; Young, M.R.; Kim, Y.S. Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev. Res. 2016, 9, 607–615. [Google Scholar] [CrossRef]
- Son, Y.; An, Y.; Jung, J.; Shin, S.; Park, I.; Gwak, J.; Ju, B.G.; Chung, Y.-H.; Na, M.; Oh, S. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53. Phytother. Res. 2019, 33, 1689–1696. [Google Scholar] [CrossRef]
- Racoma, I.O.; Meisen, W.H.; Wang, Q.-E.; Kaur, B.; Wani, A.A. Thymoquinone Inhibits Autophagy and Induces Cathepsin-Mediated, Caspase-Independent Cell Death in Glioblastoma Cells. PLoS ONE 2013, 8, e72882. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Hossain, M.K.; Mostofa, A.G.M.; Akbor, M.M.; Bin Sayeed, M.S. Therapeutic potential of thymoquinone in glioblastoma treatment: Targeting major gliomagenesis signaling pathways. BioMed Res. Int. 2018, 2018, 4010629. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Wang, C.; Mody, A.; Bao, L.; Hung, S.-H.; Svoronos, S.A.; Tseng, Y. The Effect of Z-Ligustilide on the Mobility of Human Glioblastoma T98G Cells. PLoS ONE 2013, 8, e66598. [Google Scholar] [CrossRef]
- Wu, N.; Wu, G.-c.; Hu, R.; Li, M.; Feng, H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol. Sin. 2011, 32, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jia, G.; Wang, Q.; Wang, R.; Deng, D.; Xue, L.; Shao, N.; Zhang, Y.; Xia, X.; Zhi, F. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. OncoTargets Ther. 2015, 8, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Pudełek, M.; Catapano, J.; Kochanowski, P.; Mrowiec, K.; Janik-Olchawa, N.; Czyż, J.; Ryszawy, D. Therapeutic potential of monoterpene α-thujone, the main compound of Thuja occidentalis L. essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia 2019, 134, 172–181. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhu, J.; Xu, J.; Ding, K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014, 450, 247–254. [Google Scholar] [CrossRef]
- Sales, L.; Pezuk, J.A.; Borges, K.S.; Brassesco, M.S.; Scrideli, C.A.; Tone, L.G.; dos Santos, M.H.; Ionta, M.; de Oliveira, J.C. Anticancer activity of 7-epiclusianone, a benzophenone from Garcinia brasiliensis, in glioblastoma. BMC Complement. Altern. Med. 2015, 15, 393. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fu, X.-T.; Li, Y.; Hou, Y.-J.; Yang, M.-F.; Sun, J.-Y.; Yi, S.-Y.; Fan, C.-D.; Fu, X.-Y.; Zhai, J.; et al. Induction of S-Phase Arrest in Human Glioma Cells by Selenocysteine, a Natural Selenium-Containing Agent Via Triggering Reactive Oxygen Species-Mediated DNA Damage and Modulating MAPKs and AKT Pathways. Neurochem. Res. 2016, 41, 1439–1447. [Google Scholar] [CrossRef]
- Khan, M.; Yi, F.; Rasul, A.; Li, T.; Wang, N.; Gao, H.; Gao, R.; Ma, T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life 2012, 64, 783–794. [Google Scholar] [CrossRef]
- Wang, C.-N.; Shiao, Y.-J.; Lin, Y.-L.; Chen, C.-F. Nepalolide A inhibits the expression of inducible nitric oxide synthase by modulating the degradation of IκB-α and IκB-β in C6 glioma cells and rat primary astrocytes. Br. J. Pharmacol. 1999, 128, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Tang, H.; Wang, F.; Ou, S.; Wu, T.; Fang, Y.; Xu, J.; Guo, K. Cyclovirobuxine D inhibits cell proliferation and migration and induces apoptosis in human glioblastoma multiforme and low-grade glioma. Oncol. Rep. 2020, 43, 807–816. [Google Scholar] [CrossRef]
- Guimarães, L.P.T.P.; da Graça Rocha, G.; Queiroz, R.M.; Martins, C.A.; Takiya, C.M.; Gattass, C.R. Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells. Oncol. Rep. 2017, 38, 2525–2534. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-S.; Luo, P.; Dai, S.-H.; Liu, Z.-B.; Zheng, X.-R.; Chen, T. Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell. Mol. Neurobiol. 2013, 33, 921–928. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Shiraishi, D.; Yoshitomi, M.; Ikeda, T.; Mizuta, H.; Takeya, M.; Komohara, Y. Soyasapogenols contained in soybeans suppress tumour progression by regulating macrophage differentiation into the protumoural phenotype. J. Funct. Foods 2015, 19, 594–605. [Google Scholar] [CrossRef]
- Giacomelli, C.; Daniele, S.; Natali, L.; Iofrida, C.; Flamini, G.; Braca, A.; Trincavelli, M.L.; Martini, C. Carnosol controls the human glioblastoma stemness features through the epithelial-mesenchymal transition modulation and the induction of cancer stem cell apoptosis. Sci. Rep. 2017, 7, 15174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomelli, C.; Natali, L.; Trincavelli, M.L.; Daniele, S.; Bertoli, A.; Flamini, G.; Braca, A.; Martini, C. New insights into the anticancer activity of carnosol: p53 reactivation in the U87MG human glioblastoma cell line. Int. J. Biochem. Cell Biol. 2016, 74, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, H.I.C.; Toyang, N.J.; Watson, C.T.; Ayeah, K.N.; Bryant, J. HLBT-100: A highly potent anti-cancer flavanone from Tillandsia recurvata (L.) L. Cancer Cell Int. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Pan, X.; Georgakilas, A.G.; Chen, P.; Hu, H.; Yang, Y.; Tian, S.; Xia, L.; Zhang, J.; Cai, X.; et al. Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits glioma by down regulating chemokine receptor CXCR4 expression. Cancer Lett. 2013, 336, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-B.; Zhou, L.-Z.; Mei, L.; Shi, X.-J.; Wang, X.-S.; Li, Q.-L.; Huang, L. Gambogenic acid-induced time- and dose-dependent growth inhibition and apoptosis involving Akt pathway inactivation in U251 glioblastoma cells. J. Nat. Med. 2012, 66, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.A.; Bhattacharya, S.; Elmayan, A.; Darjatmoko, S.R.; Thuro, B.A.; Yan, M.B.; van Ginkel, P.R.; Polans, A.S.; Kuo, J.S. Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J. Neurosurg. 2017, 126, 1448–1460. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Gao, Y.-Q.; Wang, X.-M.; Wang, Y.-C.; Fu, L.-Q. Germacrone inhibits the proliferation of glioma cells by promoting apoptosis and inducing cell cycle arrest. Mol. Med. Rep. 2014, 10, 1046–1050. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jiang, Z.; Ma, H.; Ning, L.; Chen, H.; Li, L.; Qi, H. Volatile Oil of Acori Graminei Rhizoma-Induced Apoptosis and Autophagy are dependent on p53 Status in Human Glioma Cells. Sci. Rep. 2016, 6, 21148. [Google Scholar] [CrossRef]
- Miao, J.; Jiang, Y.; Wang, D.; Zhou, J.; Fan, C.; Jiao, F.; Liu, B.; Zhang, J.; Wang, Y.; Zhang, Q. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/β-catenin signaling pathway. Oncol. Rep. 2015, 34, 2845–2852. [Google Scholar] [CrossRef] [Green Version]
- Nan, Y.-N.; Zhu, J.-Y.; Tan, Y.; Zhang, Q.; Jia, W.; Hua, Q. Staurosporine induced apoptosis rapidly downregulates TDP-43 in glioma cells. Asian Pac. J. Cancer Prev. 2014, 15, 3575–3579. [Google Scholar] [CrossRef] [Green Version]
- Guerram, M.; Jiang, Z.-Z.; Sun, L.; Zhu, X.; Zhang, L.-Y. Antineoplastic effects of deoxypodophyllotoxin, a potent cytotoxic agent of plant origin, on glioblastoma U-87 MG and SF126 cells. Pharmacol. Rep. 2015, 67, 245–252. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Zhang, Y.; Li, J.; Li, B.; Gao, Z.; Wang, X.; Cheng, G.; Fei, Z. Saponin B, a novel cytostatic compound purified from Anemone taipaiensis, induces apoptosis in a human glioblastoma cell line. Int. J. Mol. Med. 2013, 32, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Shu, G.; Mi, X.; Cai, J.; Zhang, X.; Yin, W.; Yang, X.; Li, Y.; Chen, L.; Deng, X. Brucine, an alkaloid from seeds of Strychnos nux-vomica Linn., represses hepatocellular carcinoma cell migration and metastasis: The role of hypoxia inducible factor 1 pathway. Toxicol. Lett. 2013, 222, 91–101. [Google Scholar] [CrossRef]
- Hsueh, K.-C.; Lin, C.-L.; Tung, J.-N.; Yang, S.-F.; Hsieh, Y.-H. Nimbolide induced apoptosis by activating ERK-mediated inhibition of c-IAP1 expression in human hepatocellular carcinoma cells. Environ. Toxicol. 2018, 33, 913–922. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Tao, S.; Sun, S. Geniposide plays anti-tumor effects by down-regulation of microRNA-224 in HepG2 and Huh7 cell lines. Exp. Mol. Pathol. 2020, 112, 104349. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, H.; Yang, H.; Huang, W.; Wu, Y.; Wang, Y.; Luo, Y.; Wang, D.; Shao, G. Solamargine triggers hepatoma cell death through apoptosis. Oncol. Lett. 2015, 10, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Hou, J.; Yin, Y.; Jang, J.; Zheng, Z.; Fan, H.; Zou, G. α-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFκB. Biochem. Pharmacol. 2010, 80, 247–254. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Tai, J.; Wang, L. Ganoderic acid A inhibits proliferation and invasion, and promotes apoptosis in human hepatocellular carcinoma cells. Mol. Med. Rep. 2017, 16, 3894–3900. [Google Scholar] [CrossRef] [Green Version]
- Law, B.Y.K.; Mok, S.W.F.; Chan, W.K.; Xu, S.W.; Wu, A.G.; Yao, X.J.; Wang, J.R.; Liu, L.; Wong, V.K.W. Hernandezine, a novel AMPK activator induces autophagic cell death in drug-resistant cancers. Oncotarget 2016, 7, 8090–8104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, H.; Wang, L.; Tan, R.; Zhu, M.; Zhong, X.; Zhang, Y.; Chen, B.; Wang, L. Decursin inhibits the growth of HepG2 hepatocellular carcinoma cells via Hippo/YAP signaling pathway. Phytother. Res. 2018, 32, 2456–2465. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; de Saeger, C.; de Bravo, M.G.; Crespo, R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018, 199, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-M.; Yang, C.-W.; Lee, Y.-Z.; Chuang, T.-H.; Wu, P.-L.; Chao, Y.-S.; Lee, S.-J. Tylophorine arrests carcinoma cells at G1 phase by downregulating cyclin A2 expression. Biochem. Biophys. Res. Commun. 2009, 386, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-J.; Lu, L.; Yang, J.-J.; Wang, X.-X.; Su, G.; Wang, Z.-l.; Chen, G.-h.; Sun, H.-m.; Wang, M.-y.; Yang, Y. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur. J. Pharmacol. 2018, 821, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-N.; Meng, X.-S.; Bao, Y.-R.; Wang, S.; Li, T.-J. Evidence for the involvement of COX-2/VEGF and PTEN/Pl3K/AKT pathway the mechanism of oroxin B treated liver cancer. Pharmacogn. Mag. 2018, 14, 207–213. [Google Scholar] [PubMed]
- Zhang, Z.; Liu, T.; Yu, M.; Li, K.; Li, W. The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 7. [Google Scholar] [CrossRef] [Green Version]
- Thirusangu, P.; Vigneshwaran, V.; Avin, B.V.; Rakesh, H.; Vikas, H.M.; Prabhakar, B.T. Scutellarein antagonizes the tumorigenesis by modulating cytokine VEGF mediated neoangiogenesis and DFF-40 actuated nucleosomal degradation. Biochem. Biophys. Res. Commun. 2017, 484, 85–92. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C.; Qian, Y.; Zhu, G. Chelerythrine inhibits human hepatocellular carcinoma metastasis in vitro. Biol. Pharm. Bull. 2018, 41, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-W.; Liu, C.-Y.; Du, C.-M.; Zhang, J.; Wu, W.-Q.; Gu, Z.-L. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J. Ethnopharmacol. 2009, 123, 293–301. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Liu, T.; Li, S.; Feng, J.; Yu, Q.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019, 8, 4806–4820. [Google Scholar] [CrossRef]
- Chan, K.T.; Meng, F.Y.; Li, Q.; Ho, C.Y.; Lam, T.S.; To, Y.; Lee, W.H.; Li, M.; Chu, K.H.; Toh, M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010, 294, 118–124. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Wu, C.-C.; Chuang, Y.-H.; Chuang, W.-L. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci. 2013, 93, 323–328. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Sindhu, G. Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed. Pharmacother. 2017, 92, 661–671. [Google Scholar] [CrossRef]
- Si, L.; Zheng, L.; Xu, L.; Yin, L.; Han, X.; Qi, Y.; Xu, Y.; Wang, C.; Peng, J. Dioscin suppresses human laryngeal cancer cells growth via induction of cell-cycle arrest and MAPK-mediated mitochondrial-derived apoptosis and inhibition of tumor invasion. Eur. J. Pharmacol. 2016, 774, 105–117. [Google Scholar] [CrossRef]
- Huang, T.-T.; Liu, F.-G.; Wei, C.-F.; Lu, C.-C.; Chen, C.-C.; Lin, H.-C.; Ojcius, D.M.; Lai, H.-C. Activation of Multiple Apoptotic Pathways in Human Nasopharyngeal Carcinoma Cells by the Prenylated Isoflavone, Osajin. PLoS ONE 2011, 6, e18308. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Zhou, H.-F. Esculetin Inhibits Proliferation, Invasion, and Migration of Laryngeal Cancer In Vitro and In Vivo by Inhibiting Janus Kinas (JAK)-Signal Transducer and Activator of Transcription-3 (STAT3) Activation. Med. Sci. Monit. 2019, 25, 7853–7863. [Google Scholar] [CrossRef]
- Cheng, M.-F.; Lin, C.-S.; Chen, Y.-H.; Sung, P.-J.; Lin, S.-R.; Tong, Y.-W.; Weng, C.-F. Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar. Drugs 2017, 15, 224. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Yu, X.; Xiao, L.; Hu, Z.; Luo, X.; Tao, Y.; Yang, L.; Liu, X.; Chen, H.; Ding, Z.; et al. Neoalbaconol induces energy depletion and multiple cell death in cancer cells by targeting PDK1-PI3-K/Akt signaling pathway. Cell Death Dis. 2013, 4, e804. [Google Scholar] [CrossRef] [Green Version]
- Kowshik, J.; Nivetha, R.; Ranjani, S.; Venkatesan, P.; Selvamuthukumar, S.; Veeravarmal, V.; Nagini, S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life 2019, 71, 1595–1610. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Hsieh, M.-J.; Yang, J.-S.; Yang, S.-F.; Chuang, Y.-T.; Su, S.-C.; Liang, M.-Y.; Chen, M.-K.; Lin, C.-W. Geraniin inhibits oral cancer cell migration by suppressing matrix metalloproteinase-2 activation through the FAK/Src and ERK pathways. Environ. Toxicol. 2019, 34, 1085–1093. [Google Scholar] [CrossRef]
- He, J.; Wei, W.; Yang, Q.; Wang, Y. Phillygenin Exerts In Vitro and In Vivo Antitumor Effects in Drug-Resistant Human Esophageal Cancer Cells by Inducing Mitochondrial-Mediated Apoptosis, ROS Generation, and Inhibition of the Nuclear Factor kappa B NF-κB Signalling Pathway. Med. Sci. Monit. 2019, 25, 739–745. [Google Scholar] [CrossRef]
- Wu, G.; Chen, G.; Zhou, J.; Zhu, H.; Chu, J.; Zhang, F. Liriodenine enhances radiosensitivity in esophageal cancer ECA-109 cells by inducing apoptosis and G2/M arrest. Oncol. Lett. 2018, 16, 5020–5026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-W.; Bai, L.-Y.; Su, J.-H.; Chiu, C.-F.; Lin, W.-Y.; Huang, W.-T.; Shih, M.-C.; Huang, Y.-T.; Hu, J.-L.; Weng, J.-R. Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines 2020, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Rizo, W.F.; Ferreira, L.E.; Colnaghi, V.; Martins, J.S.; Franchi, L.P.; Takahashi, C.S.; Beleboni, R.O.; Marins, M.; Pereira, P.S.; Fachin, A.L. Cytotoxicity and genotoxicity of coronaridine from Tabernaemontana catharinensis A. DC in a human laryngeal epithelial carcinoma cell line (Hep-2). Genet. Mol. Biol. 2013, 36, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, R.; Skaf, J.; Roller, J.; Polednik, C.; Holzgrabe, U.; Schmidt, M. Anticancer effects of NSC-631570 (Ukrain) in head and neck cancer cells: In vitro analysis of growth, invasion, angiogenesis and gene expression. Oncol. Rep. 2020, 43, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.R.; Li, Y.; Upadhyay, S.; Koppolu, P.K.; Sarkar, F.H. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 2001, 20, 2927–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef]
- Bell, C.; Hawthorne, S. Ellagic acid, pomegranate and prostate cancer—A mini review. J. Pharm. Pharmacol. 2008, 60, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Eskandari, E.; Heidarian, E.; Amini, S.; Saffari-Chaleshtori, J. Evaluating the effects of ellagic acid on pSTAT3, pAKT, and pERK1/2 signaling pathways in prostate cancer PC3 cells. J. Cancer Res. Ther. 2016, 12, 1266–1271. [Google Scholar]
- Lee, H.-S.; Safe, S.; Lee, S.-O. Inactivation of the orphan nuclear receptor NR4A1 contributes to apoptosis induction by fangchinoline in pancreatic cancer cells. Toxicol. Appl. Pharmacol. 2017, 332, 32–39. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, J.-H.; Fang, L. A novel monoterpenoid indole alkaloid with anticancer activity from Melodinus khasianus. Bioorg. Med. Chem. Lett. 2017, 27, 893–896. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Jayasooriya, R.G.P.T.; Choi, Y.H.; Kim, G.-Y. Camptothecin induces c-Myc- and Sp1-mediated hTERT expression in LNCaP cells: Involvement of reactive oxygen species and PI3K/Akt. Food Chem. Toxicol. 2019, 127, 53–60. [Google Scholar] [CrossRef]
- Forestier-Román, I.S.; López-Rivas, A.; Sánchez-Vázquez, M.M.; Rohena-Rivera, K.; Nieves-Burgos, G.; Ortiz-Zuazaga, H.; Torres-Ramos, C.A.; Martínez-Ferrer, M. Andrographolide induces DNA damage in prostate cancer cells. Oncotarget 2019, 10, 1085–1101. [Google Scholar] [CrossRef] [Green Version]
- Nie, C.; Zhou, J.; Qin, X.; Shi, X.; Zeng, Q.; Liu, J.; Yan, S.; Zhang, L. Diosgenin-induced autophagy and apoptosis in a human prostate cancer cell line. Mol. Med. Rep. 2016, 14, 4349–4359. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Amin, K.S.; Jagadeesh, S.; Baishay, G.; Rao, P.G.; Barua, N.C.; Bhattacharya, S.; Banerjee, P.P. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol. Cancer 2013, 12, 99. [Google Scholar] [CrossRef]
- Shang, X.J.; Yao, G.; Ge, J.P.; Sun, Y.; Teng, W.H.; Huang, Y.F. Procyanidin Induces Apoptosis and Necrosis of Prostate Cancer Cell Line PC-3 in a Mitochondrion-Dependent Manner. J. Androl. 2009, 30, 122–126. [Google Scholar] [CrossRef]
- Bommareddy, A.; McGlynn, D.; Lewis, M.; Lockus, L.; Seward, J.; Hong, K.L.; VanWert, A.L.; Dwivedi, C. Akt/survivin pathway inhibition enhances the apoptotic cell death-induced by alpha-santalol in human prostate cancer cells. Fitoterapia 2020, 143, 104552. [Google Scholar] [CrossRef]
- Jiang, C.; Masood, M.; Rasul, A.; Wei, W.; Wang, Y.; Ali, M.; Mustaqeem, M.; Li, J.; Li, X. Altholactone Inhibits NF-κB and STAT3 Activation and Induces Reactive Oxygen Species-Mediated Apoptosis in Prostate Cancer DU145 Cells. Molecules 2017, 22, 240. [Google Scholar] [CrossRef] [Green Version]
- Liew, S.Y.; Looi, C.Y.; Paydar, M.; Cheah, F.K.; Leong, K.H.; Wong, W.F.; Mustafa, M.R.; Litaudon, M.; Awang, K. Subditine, a New Monoterpenoid Indole Alkaloid from Bark of Nauclea subdita (Korth.) Steud. Induces Apoptosis in Human Prostate Cancer Cells. PLoS ONE 2014, 9, e87286. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, Y.; Liang, C. Eriocalyxin B induces apoptosis and autophagy involving akt/mammalian target of rapamycin (mTOR) pathway in prostate cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8534–8543. [Google Scholar] [CrossRef]
- Wang, Y.; Tsai, M.-L.; Chiou, L.-Y.; Ho, C.-T.; Pan, M.-H. Antitumor Activity of Garcinol in Human Prostate Cancer Cells and Xenograft Mice. J. Agric. Food Chem. 2015, 63, 9047–9052. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Lorigooini, Z.; Wibowo, J.; Amini-khoei, H. Tricin isolated from Allium atroviolaceum potentiated the effect of docetaxel on PC3 cell proliferation: Role of miR-21. Nat. Prod. Res. 2019, 33, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Q.; Wang, Z.; Cao, X. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed. Pharmacother. 2019, 112, 108592. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-H.; Zhou, H.-J.; Lee, J. Dihydroartemisinin inhibits angiogenesis induced by multiple myeloma RPMI8226 cells under hypoxic conditions via downregulation of vascular endothelial growth factor expression and suppression of vascular endothelial growth factor secretion. Anti-Cancer Drugs 2006, 17, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.-C.; Shih, Y.-L.; Chou, J.-S.; Chen, K.-W.; Chen, Y.-L.; Lee, M.-H.; Peng, S.-F.; Leu, S.-J.; Chung, J.-G. Cardamonin induces cell cycle arrest, apoptosis and alters apoptosis associated gene expression in WEHI-3 mouse leukemia cells. Am. J. Chin. Med. 2019, 47, 635–656. [Google Scholar] [CrossRef]
- Martins, C.; Doran, C.; Silva, I.C.; Miranda, C.; Rueff, J.; Rodrigues, A.S. Myristicin from nutmeg induces apoptosis via the mitochondrial pathway and down regulates genes of the DNA damage response pathways in human leukaemia K562 cells. Chem.-Biol. Interact. 2014, 218, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.-F.; Xiao, Z.-J.; Wang, H.-H.; Zhou, J.-M.; Mei, Y.-P.; Deng, R.; Jiang, W.-Q.; Liu, Z.-C. Inducement effect of Meisoindigo on apoptosis of leukemia cell line HL-60 and its mechanism. Ai Zheng Chin. J. Cancer 2005, 24, 1464–1468. [Google Scholar]
- Kluza, J.; Mazinghien, R.; Degardin, K.; Lansiaux, A.; Bailly, C. Induction of apoptosis by the plant alkaloid sampangine in human HL-60 leukemia cells is mediated by reactive oxygen species. Eur. J. Pharmacol. 2005, 525, 32–40. [Google Scholar] [CrossRef]
- Saeed, M.; Jacob, S.; Sandjo, L.P.; Sugimoto, Y.; Khalid, H.E.; Opatz, T.; Thines, E.; Efferth, T. Cytotoxicity of the Sesquiterpene Lactones Neoambrosin and Damsin from Ambrosia maritima Against Multidrug-Resistant Cancer Cells. Front. Pharmacol. 2015, 6, 267. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Mazza, T.; Morozzi, G.; Fabiani, R. Pinoresinol Inhibits Proliferation and Induces Differentiation on Human HL60 Leukemia Cells. Nutr. Cancer 2013, 65, 1208–1218. [Google Scholar] [CrossRef]
- Gaboriaud-Kolar, N.; Myrianthopoulos, V.; Vougogiannopoulou, K.; Gerolymatos, P.; Horne, D.A.; Jove, R.; Mikros, E.; Nam, S.; Skaltsounis, A.-L. Natural-based indirubins display potent cytotoxicity toward wild-type and t315i-resistant leukemia cell lines. J. Nat. Prod. 2016, 79, 2464–2471. [Google Scholar] [CrossRef]
- Hoffmann, R.; von Schwarzenberg, K.; López-Antón, N.; Rudy, A.; Wanner, G.; Dirsch, V.M.; Vollmar, A.M. Helenalin bypasses Bcl-2-mediated cell death resistance by inhibiting NF-κB and promoting reactive oxygen species generation. Biochem. Pharmacol. 2011, 82, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Karmahapatra, S.; Kientz, C.; Shetty, S.; Yalowich, J.C.; Rakotondraibe, L.H. Capsicodendrin from Cinnamosma fragrans Exhibits Antiproliferative and Cytotoxic Activity in Human Leukemia Cells: Modulation by Glutathione. J. Nat. Prod. 2018, 81, 625–629. [Google Scholar] [CrossRef]
- Li, C.; Dong, L.; Su, R.; Bi, Y.; Qing, Y.; Deng, X.; Zhou, Y.; Hu, C.; Yu, M.; Huang, H.; et al. Homoharringtonine exhibits potent anti-tumor effect and modulates DNA epigenome in acute myeloid leukemia by targeting SP1/TET1/5hmC. Haematolgica 2020, 105, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Sung, B.; Ahn, K.S.; Aggarwal, B.B. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-κB signaling pathway. Cancer Res. 2010, 70, 3259–3268. [Google Scholar] [CrossRef] [Green Version]
- Yusenko, M.V.; Trentmann, A.; Andersson, M.K.; Ghani, L.A.; Jakobs, A.; Arteaga Paz, M.-F.A.; Mikesch, J.-H.; von Kries, J.P.; Stenman, G.; Klempnauer, K.-H. Monensin, a novel potent MYB inhibitor, suppresses proliferation of acute myeloid leukemia and adenoid cystic carcinoma cells. Cancer Lett. 2020, 479, 61–70. [Google Scholar] [CrossRef]
- Issa, M.E.; Berndt, S.; Carpentier, G.; Pezzuto, J.M.; Cuendet, M. Bruceantin inhibits multiple myeloma cancer stem cell proliferation. Cancer Biol. Ther. 2016, 17, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Alachkar, H.; Santhanam, R.; Harb, J.G.; Lucas, D.M.; Oaks, J.J.; Hickey, C.J.; Pan, L.; Kinghorn, A.D.; Caligiuri, M.A.; Perrotti, D.; et al. Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia. J. Hematol. Oncol. 2013, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gong, L.; Qi, R.; Sun, Q.; Xia, X.; He, H.; Ren, J.; Zhu, O.; Zhuo, D. Paeoniflorin suppresses pancreatic cancer cell growth by upregulating HTRA3 expression. Drug Des. Dev. Ther. 2017, 11, 2481–2491. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Wang, L.; Lu, A.; Liu, X.; Sang, M.; Meng, F.; Cao, Q.; Ji, X. The flavonoid Baohuoside-I inhibits cell growth and downregulates survivin and cyclin D1 expression in esophageal carcinoma via β-catenin-dependent signaling. Oncol. Rep. 2011, 26, 1149–1156. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.G.; Chung, J.-Y.; Kim, Y.-J.; Park, J.-E.; Koh, H.; Han, M.S.; Park, Y.C.; Yoo, Y.H.; Kim, J.-M. Ellipticine induces apoptosis in human endometrial cancer cells: The potential involvement of reactive oxygen species and mitogen-activated protein kinases. Toxicology 2011, 289, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Zhang, Y.-q.; Ge, L.-t.; Chen, J.; Jia, X.-q.; Gu, R.-x.; Sun, Y.; Sun, W.-d. Ilexgenin A induces B16-F10 melanoma cell G1/S arrest in vitro and reduces tumor growth in vivo. Int. Immunopharmacol. 2015, 24, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Saisomboon, S.; Kariya, R.; Vaeteewoottacharn, K.; Wongkham, S.; Sawanyawisuth, K.; Okada, S. Antitumor effects of flavopiridol, a cyclin-dependent kinase inhibitor, on human cholangiocarcinoma in vitro and in an in vivo xenograft model. Heliyon 2019, 5, e01675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khumkhrong, P.; Piboonprai, K.; Chaichompoo, W.; Pimtong, W.; Khongkow, M.; Namdee, K.; Jantimaporn, A.; Japrung, D.; Asawapirom, U.; Suksamrarn, A.; et al. Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells. Biomolecules 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Tang, B.; Wang, J.; Sui, H.; Jin, X.; Wang, L.; Wang, Z. Antiproliferative effect of alpinetin in BxPC-3 pancreatic cancer cells. Int. J. Mol. Med. 2012, 29, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Lai, Y.; Zhu, M.; Huang, S.; Feng, W.; Gu, X. Combretastatin A4 Regulates Proliferation, Migration, Invasion, and Apoptosis of Thyroid Cancer Cells via PI3K/Akt Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4911–4917. [Google Scholar] [CrossRef]
- Appadurai, P.; Rathinasamy, K. Indicine N-oxide binds to tubulin at a distinct site and inhibits the assembly of microtubules: A mechanism for its cytotoxic activity. Toxicol. Lett. 2014, 225, 66–77. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, Q.; Zhao, G.; Zhang, J.; Yuan, H.; Gong, D.; Guo, Y.; Liu, X.; Li, K.; Lin, P. Alkannin induces cytotoxic autophagy and apoptosis by promoting ROS-mediated mitochondrial dysfunction and activation of JNK pathway. Biochem. Pharmacol. 2020, 180, 114167. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, J.-H.; Ahn, J.-H.; Lee, K.-T.; Baek, N.-I.; Choi, J.-H. Jaceosidin, isolated from dietary mugwort (Artemisia princeps), induces G2/M cell cycle arrest by inactivating cdc25C-cdc2 via ATM-Chk1/2 activation. Food Chem. Toxicol. 2013, 55, 214–221. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, H.; Nakamura, S.; Chisaki, Y.; Takada, T.; Toda, Y.; Murata, H.; Itoh, K.; Yano, Y.; Takata, K.; Ashihara, E. Daphnetin inhibits invasion and migration of LM8 murine osteosarcoma cells by decreasing RhoA and Cdc42 expression. Biochem. Biophys. Res. Commun. 2016, 471, 63–67. [Google Scholar] [CrossRef]
- Hossan, M.S.; Chan, Z.-Y.; Collins, H.M.; Shipton, F.N.; Butler, M.S.; Rahmatullah, M.; Lee, J.B.; Gershkovich, P.; Kagan, L.; Khoo, T.-J.; et al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/mTOR signal transduction inhibition. Cancer Lett. 2019, 453, 57–73. [Google Scholar] [CrossRef]
- Deng, X.; Sheng, J.; Liu, H.; Wang, N.; Dai, C.; Wang, Z.; Zhang, J.; Zhao, J.; Dai, E. Cinobufagin Promotes Cell Cycle Arrest and Apoptosis to Block Human Esophageal Squamous Cell Carcinoma Cells Growth via the p73 Signalling Pathway. Biol. Pharm. Bull. 2019, 42, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, L.; Li, J.; Zhang, G.; Tao, H.; Li, X.; Sun, D.; Hu, Y. Swainsonine Inhibits Invasion and the EMT Process in Esophageal Carcinoma Cells by Targeting Twist1. Oncol. Res. 2018, 26, 1207–1213. [Google Scholar] [CrossRef]
- Lu, C.-H.; Chen, S.-H.; Chang, Y.-S.; Liu, Y.-W.; Wu, J.-Y.; Lim, Y.-P.; Yu, H.-I.; Lee, Y.-R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef]
- Rabi, T.; Catapano, C.V. Aphanin, a triterpenoid from Amoora rohituka inhibits K-Ras mutant activity and STAT3 in pancreatic carcinoma cells. Tumor Biol. 2016, 37, 12455–12464. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; de Andrade, C.M. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Mansingh, D.P.; OJ, S.; Sali, V.K.; Vasanthi, H.R. [6]-Gingerol–induced cell cycle arrest, reactive oxygen species generation, and disruption of mitochondrial membrane potential are associated with apoptosis in human gastric cancer (AGS) cells. J. Biochem. Mol. Toxicol. 2018, 32, e22206. [Google Scholar] [CrossRef]
- Yong, W.K.; Abd Malek, S.N. Xanthohumol Induces Growth Inhibition and Apoptosis in Ca Ski Human Cervical Cancer Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 921306. [Google Scholar] [CrossRef] [Green Version]
- An, F.; Wang, S.; Tian, Q.; Zhu, D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol. Lett. 2015, 10, 2627–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yu, Y.; Zhang, Q.; Li, X.; Zhang, C.; Mao, T.; Liu, S.; Tian, Z. Anti-gastric cancer effect of Salidroside through elevating miR-99a expression. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3500–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Júnior, P.L.d.S.; Câmara, D.A.D.; Costa, A.S.; Ruiz, J.L.M.; Levy, D.; Azevedo, R.A.; Pasqualoto, K.F.M.; de Oliveira, C.F.; de Melo, T.C.; Pessoa, N.D.S.; et al. Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine 2016, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P.; Seukep, J.A.; Zeino, M.; Mbaveng, A.T.; Ngadjui, B.; Efferth, T. Cytotoxic compounds from the fruits of Uapaca togoensis towards multifactorial drug-resistant cancer cells. Planta Med. 2015, 81, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Wang, C.; Yi, X.; Li, M.; He, X. Anticancer activities of harmine by inducing a pro-death autophagy and apoptosis in human gastric cancer cells. Phytomedicine 2017, 28, 10–18. [Google Scholar] [CrossRef]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef]
- Edler, M.C.; Fernandez, A.M.; Lassota, P.; Ireland, C.M.; Barrows, L.R. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002, 63, 707–715. [Google Scholar] [CrossRef]
- Zou, P.; Xia, Y.; Ji, J.; Chen, W.; Zhang, J.; Chen, X.; Rajamanickam, V.; Chen, G.; Wang, Z.; Chen, L.; et al. Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer. Cancer Lett. 2016, 375, 114–126. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, Y.; Ma, J.; Ou, W.; Wang, H.; Wu, J.; Tang, J.; Zhang, B.; Liao, X.; Yang, D.; et al. Parthenolide Inhibits Angiogenesis in Esophageal Squamous Cell Carcinoma Through Suppression of VEGF. OncoTargets Ther. 2020, 13, 7447–7458. [Google Scholar] [CrossRef]
- Zhao, Z.; Jia, Q.; Wu, M.-S.; Xie, X.; Wang, Y.; Song, G.; Zou, C.-Y.; Tang, Q.; Lu, J.; Huang, G.; et al. Degalactotigonin, a natural compound from Solanum nigrum L., inhibits growth and metastasis of osteosarcoma through GSK3β inactivation—Mediated repression of the Hedgehog/Gli1 pathway. Clin. Cancer Res. 2018, 24, 130–144. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural Product-Based Nanoformulations for Cancer Therapy: Opportunities and Challenges. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Nanobiomaterials in Cancer Therapy. In Nanobiomaterials in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–89. [Google Scholar]
- Mousa, D.S.; El-Far, A.H.; Saddiq, A.A.; Sudha, T.; Mousa, S.A. Nanoformulated Bioactive Compounds Derived from Different Natural Products Combat Pancreatic Cancer Cell Proliferation. Int. J. Nanomed. 2020, 15, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Andima, M.; Costabile, G.; Isert, L.; Ndakala, A.J.; Derese, S.; Merkel, O.M. Evaluation of β-Sitosterol loaded PLGA and PEG-PLA nanoparticles for effective treatment of breast cancer: Preparation, physicochemical characterization, and antitumor activity. Pharmaceutics 2018, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Xu, M.; Wang, J.; Zhou, S.; Liu, Y.; Liu, S.; Huang, Y.; Chen, Y.; Chen, L.; Song, Q.; et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials 2020, 241, 119907. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C 2016, 64, 124–132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Shen, G.; Chang, R.; Zhang, Y.; Yan, X. Coordination self-assembly of natural flavonoids into robust nanoparticles for enhanced in vitro chemo and photothermal cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124805. [Google Scholar] [CrossRef]
- Ahmadi, E.; Zarghami, N.; Jafarabadi, M.A.; Alizadeh, L.; Khojastehfard, M.; Yamchi, M.R.; Salehi, R. Enhanced anticancer potency by combination chemotherapy of HT-29 cells with biodegradable, pH-sensitive nanoparticles for co-delivery of hydroxytyrosol and doxorubicin. J. Drug Deliv. Sci. Technol. 2019, 51, 721–735. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Samaha, M.W.; Fang, J.-Y.; Freag, M.S. Liquid crystalline assembly for potential combinatorial chemo–herbal drug delivery to lung cancer cells. Int. J. Nanomed. 2019, 14, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Ling, L.; Ismail, M.; Du, Y.; He, W.; Zhou, W.; Yao, C.; Li, X. Paclitaxel encapsulated in artesunate-phospholipid liposomes for combinatorial delivery. J. Drug Deliv. Sci. Technol. 2019, 51, 372–382. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.; Guo, D. Targeted Therapy for Hepatocellular Carcinoma: Co-Delivery of Sorafenib and Curcumin Using Lactosylated pH-Responsive Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 647. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.; et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Muhammad, N.; Li, T.; Wang, H.; Liu, Y.; Liu, B.; Zhan, H. Hyaluronic Acid-Coated Camptothecin Nanocrystals for Targeted Drug Delivery to Enhance Anticancer Efficacy. Mol. Pharm. 2020, 17, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liang, T.; Min, Q.; Jiang, L.-P.; Zhu, J.-J. “Stealth and Fully-Laden” Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9938–9948. [Google Scholar] [CrossRef]
- Amaral, R.; dos Santos, S.A.; Andrade, L.N.; Severino, P.; Carvalho, A.A. Natural Products as Treatment against Cancer: A Historical and Current Vision. Clin. Oncol. 2019, 4, 1562. [Google Scholar]
- Mita, A.; Lockhart, A.C.; Chen, T.-L.; Bochinski, K.; Curtright, J.; Cooper, W.; Hammond, L.; Rothenberg, M.; Rowinsky, E.; Sharma, S. A phase I pharmacokinetic (PK) trial of XAA296A (Discodermolide) administered every 3 wks to adult patients with advanced solid malignancies. J. Clin. Oncol. 2004, 22, 2025. [Google Scholar] [CrossRef]




| Sources | Bioactive Constituents | Chemical Formula | Molecular Weight (g/mol) | Mechanisms | Study Model | Concentrations | Other Cancers | References |
|---|---|---|---|---|---|---|---|---|
| Lung cancer | ||||||||
| Fascaplysinopsis Bergquist | Fascaplysin | C18H11ClN2O | 306.7 | ↓CDK-4, ↑ROS, ↓mTOR, ↓4EBP1, ↓p70S6K1 | A549, H1299, PC-9 and NCI-H526 cell lines | IC50: 0.57–1.15 µM | Liver, ovarian, and colon cancer | [195] |
| Chelidonium majus | Chelidonine | C20H19NO5 | 353.4 | ↓cyclin B1, ↑p21, ↓MMP, ↑ROS/AMPK, ↓p-p70S6K, ↓EGFR, G2/M phase arrest | A549, PC9, H460, H358 cells and H1975 xenograft in mice | IC50: 2.58–20 µM | Breast, liver, head, and neck cancer | [196] |
| Cephaelis Ipecacuanha | Emetine | C29H40N2O4 | 480.6 | ↓MMP-2/-9, ↓ERK1/2, and ↑p38 | A549 and H1299 cell line | IC50: 0.243 and 1 µM | Breast, colon, prostate, and pancreatic cancer | [197] |
| Citrus limon | D-limonene | C10H16 | 136.23 | ↑Bax, ↓Bcl-2, ↑cleaved PARP, ↑Atg5 | H1299 and A549 cells xenograft in Balb/c mice | 0.5–0.75 mM | Skin, liver, breast, and kidney cancer | [198] |
| Artemisia annua | Sesquiterpene (AMDT) | C17H26O2 | 262.39 | ↑caspase 3- 9, G1/S phase arrest | 95-D, HO8910, QGY, and HeLa cells | IC50: 52.44–73.3 µM | Gastric and ovarian cancer | [199] |
| Artemisinin | C15H22O5 | 282.33 | ↓VEGF-C, ↓p38 MAPK | LLC cells and C57BL/6 mice | 5–20 µM | Breast, cervical, gastric, liver, and bladder cancer | [200] | |
| Artemether | C16H26O5 | 298.37 | ↓Bcl-2, ↓cIAP1, ↓cIAP2, ↓CDK1, ↓CDK2, ↓CDK6, ↓cyclin A2, ↓cyclin B1, ↓cyclin D1, ↑p16 | A549, NCI-H1299 cells | 40–80 µM | Brain, breast, and gastric cancer | [201] | |
| Betula platyphylla | Betulin | C30H50O2 | 442.7 | ↑caspase 3- 9 and 8, ↑ Bax, ↑Cyto- C, ↑Bak, ↓PARP | A549, HepG2, Hela, MCF-7 cells | IC50: 10–15 µg/mL | Gastric, renal, colon, and melanoma | [202] |
| Betulinic Acid | C30H48O3 | 456.7 | ↓cyclin A2, ↓Sp1, G2/M phase arrest | A549, Hela, H1299 xenograft mice and Scgb1a1-rtTA/TetO-KrasG12Dmice | ≤40 µM | Colon, breast, prostate, nasopharyngeal, and cervical cancer | [203,204] | |
| Garcinia | Gambogic acid | C38H44O8 | 628.7 | ↑Caspase-3, ↓Bcl2, ↓pI3K, ↓DLL1, ↓DLL3, ↓ DLL4, ↓Jagged1, ↓Jagged2 | SPC-A-1 and A549 cells | 0.5–1 µmol/L | Liver and colorectal | [205] |
| Laminaria japonica | Fucoxanthin | C42H58O6 | 658.9 | ↑p21, ↑p53, ↑puma, ↑Fas, ↓Bcl-2, G0/G1 phase arrest | SPC-A1, A549, H460, H1299 | IC50: <82 µM | Skin, gastric, breast, and colon cancer | [206] |
| Chinese trichosanthes | Trichosanthin | 247 Amino acids | 27 kDa | ↑Caspase-3 and 9, ↑E-cadherin, ↓N-cadherin, ↓Bcl-2, ↑ Bax, ↑DR4, ↑DR5 | NCI-H1299, NCI-H1975, and A549 cells | IC20: 39.5, 61.1 and 49.3 µg/mL | Cervical, gastric, breast, and nasopharyngeal | [207] |
| Hedyotis | Ursolic acid | C30H48O3 | 456.7 | ↓MMP2, ↓MMP 9, ↑E-cadherin, ↓N-cadherin | H1975 cells | IC50: 29.7 nM | Breast, prostate, cervical, colorectal, and liver cancer | [208] |
| Oleanolic acid | C30H48O3 | 456.7 | ↑ROS, ↑Ca2+, ↓Akt, ↓JACk2, ↓STAT3 | A549 cells | IC50: 9.66 µM | Gallbladder, prostate, and gastric cancer | [209] | |
| Scutellaria barbata | Wogonin | C16H12O5 | 284.26 | ↑caspase-3, 8 and 9, ↑ROS | A427, BEAS-2B and A459 cells | 50 µM | Breast, cervical, and nasopharyngeal carcinoma | [210] |
| Luteolin | C15H10O6 | 286.24 | ↑miR-34a-5p, ↑p21, ↑p53, ↓MDM4, ↑caspase-3 and 9 | A549, and H460 xenograft model | IC50: 40 µM | Breast, ovarian, gastric, and prostate cancer | [211] | |
| Polygonum cuspidatum | Polydatin | C20H22O8 | 390.4 | ↓NLRP3, ↓ASC, ↓p-NF-κβ p65 | A549 and H1299 cells | 50 µM | Breast, hepatic, renal, and ovarian cancer | [212] |
| Rheum officinale | Emodin | C15H10O5 | 270.24 | ↑PPARγ, ↑IGFBP1, ↓Sp1 | H1975, and A549 cells xenograft mice | 50 µM | Renal and cervical cancer | [213] |
| Rhizoma zedoariae | β-elemene | C15H24 | 204.35 | ↑caspase-3, ↑ROS, ↑Cyto- C, ↑Bad, ↓Bcl-2 | A549/DDP cells | 20 and 40 µg/mL | Liver, gastric, and bladder cancer | [214] |
| Chansu | Bufalin | C24H34O4 | 386.5 | ↑caspase-3, ↓Akt, ↓ ERK ½, ↓VEGF, ↓cMyc, ↓NF-κβ, ↓p38 MAPK, G1/S phase arrest | A549, NCI-H460 | 2.5–10 µM | Prostate, liver, and ovarian cancer | [215,216] |
| Platycodon grandiflorum | Platycodin-D | C57H92O28 | 1225.3 | ↑Atg-3, ↑Atg-7, ↑Beclin-1, ↑LC3-II, ↓ p-Akt, ↓p-p70S6K, ↓p-4EBP1, ↓ ERK ½ | NCI-H460 and A549 cells | 5–30 µmol/L | Colon, leukemia, and breast cancer | [217] |
| Salvia miltiorrhiza | Tanshinone IIA (TSIIA) | C19H18O3 | 294.34 | ↑Cyto- C, ↓ Bax, ↓MMP, ↑caspase-3 and 9 | A549 cells | IC50: 14.5 µM | Prostate, glioma, leukemia, and hepatoma | [218] |
| Tripterygium wilfordii Hook | Celastrol | C29H38O4 | 450.6 | ↓Bcl-2, ↑ Bax, ↓Akt, ↑Cyto- C, ↓PARP, ↑Fas, ↑FasL | A549 cells | IC50: 2.12 µM | Prostate, brain, and liver cancer | [219] |
| Curcuma longa | Curcumin | C21H20O6 | 368.4 | ↑ROS, ↓ΔΨm, ↓SOD1, ↑caspase-3 and 9, ↑ Bax, G2/M phase arrest | A549 and SPC-A1 cells | 10–25 µM | Pancreatic, gastric, prostate, and colorectal cancer | [220,221] |
| Cinnamomum cassia | Cinnamaldehyde | C9H8O | 132.16 | ↑caspase-3, ↑PARP, ↓Bcl-2,↑Bax,↓Bcl-xL, ↓HIF-1α, ↓β-catenin, ↓MMPs, ↑E-cadherin | NCI-H1299, YTMLC, A549 xenograft model | IC50: 10.5, 32, 41 µg/mL | oral squamous cell carcinoma and colorectal cancer | [222] |
| Coix lacryma-jobi L. | CP-1 polysaccharide | n.d. | n.d. | ↑caspase-3 and 9, ↓ΔΨm, S phase arrest | A549 cells | 10–300 µg/mL | Breast and colon cancer | [223] |
| Ophiopogon japonicus | Ophiopogonin B | C39H62O12 | 722.9 | ↑E-cadherin, ↓N-cadherin, ↓linc00668, ↑miR-432-5p | A549, 293T and THP-1 cells | 5 and 10 µmol/L | Colon, breast, and gastric cancer | [224] |
| Tetrastigma hemsleyanum | Radix Tetrastigma Hemsleyani Flavone | C27H30O10 | 514.5 | ↓MMP 2 and 9, ↓TIMP-1, ↑TIMP-2 | A549 cells | 0.5–10 mg/mL | Liver, leukemia, and stomach cancer | [225] |
| Panax notoginseng | Trilinolein | C57H98O6 | 879.4 | ↓Bcl-2, ↑ Bax, ↑ROS, ↓PARP, ↓Akt, ↑caspase-3, ↑Cyto- C | A549, MKN-45 and A498 cells | 25–100 µg/mL | Liver, colon, and gastric cancer | [226] |
| Fucus vesiculosus | Fucoidan | C7H14O7S | 242.25 | ↑ROS, ↑ATF4, ↑CHOP, ↑TLR4 | A549, CL1-5 and LLC-1 xenograft C57BL/6 mice | 100–800 µg/mL | Breast, liver, and colon cancer | [227] |
| Breast cancer | ||||||||
| Rabdosia rubescens | Oridonin | C20H28O6 | 364.4 | ↓Jagged2, ↓Notch1-4 | 4T1 cells and xenograft BALB/c mice | 0.1–10 mmol/L | Gastric, esophageal, prostate, and pancreatic cancer | [228] |
| Panax notoginseng | Panaxadiol saponins | C30H52O3 | 460.73 | ↓NLR, ↓MPO, ↓G-CSF, ↓PU.1, ↓C/EBPα, ↓EP1, ↓GATA 1 and 2 | 4T1-Luc and xenograft BALB/c mice | 0.1–40 µM | Lung, liver, and colon cancer | [229] |
| Ganoderma lucidum | Ganodermanontriol | C30H48O4 | 472.7 | ↓u-PA, ↓CDC20, ↓survivin | MDA-MB-231 cells | IC50: 11.6 µM | Gastric, prostate, and colon cancer | [230] |
| Polysaccharides | n.d. | n.d. | ↑SOD, ↑CAT, ↑GPx, ↓IL-1β, ↓IL-6, ↓TNF-α | MDA-MB-231 cells, Wistar rats | 0.5–3 mg/mL | Melanoma, cervical, and colorectal cancer | [231,232] | |
| Maclura pomifera | Pomiferin | C25H24O6 | 420.5 | ↑CANX, ↑BCAP31, ↑Mn-SOD,↑ACOX1,↓BMP-7 ↓ID 2 and 3 | MCF-7 and MCF-10A | IC50: 5.2 µM | Liver, cholangiocarcinoma, and colon cancer | [233] |
| Chansu | Bufadienolides | C24H34O2 | 354.52 | ↓STAT3/Mcl-1, ↓Bcl-xL, ↓PARP, ↑caspase-3 | MDA-MB-231, MCF-10A and MCF-7 cells | 0.1–0.5 µM | Liver, leukemia, lung, and gastric cancer | [234] |
| Cimicifuga foetida | Actein | C37H56O11 | 676.8 | ↓VEGF, ↓pERK, ↓pJNK, ↓CD34, ↓CXCR4 | HMEC-1, 4T1 cells xenograft in mice | IC50: 0.065 µM | Gastric, bladder, and lung cancer | [235] |
| Astragalus membranaceus | Formononetin | C16H12O4 | 268.26 | ↓MMP 2, ↓MMP9, ↓PI3K, ↓Akt, ↑TIMP-1 and 2 | MDA-MB-231 and 4T1 cells | 2.5–160 µmol/L | Cervical, bladder, and gastric cancer | [236] |
| Astragulus Polysaccharides | n.d. | n.d. | ↓Bcl-2, ↑ Bax, ↑NO, ↑TNF-α, G1/S phase arrest | MCF-7 cells | 100–1000 µg/mL | Liver, lung, and gastric cancer | [237] | |
| Narcissus L. bulb | Narciclasine | C14H13NO7 | 307.25 | ↑AMPK-ULK1, ↓PRAS40 | MDA-MB-231, MCF-7, MCF-10A, BT483, HCC1937 and xenograft in mice | IC50: 5–100 nM | Brain, liver, and hematological cancers | [238] |
| Cimicifuga racemosa | KHF16 | C37H58O10 | 662.85 | ↓XIAP, Mcl-1, ↓Survivin, ↓Cyclin B1/D1, ↓NF-kβ | MDA-MB-231, MDA-MB-468 and SW527 | IC50: <10 µM | Gastric, bladder, and lung cancer | [239] |
| Pueraria lobata | Puerarin | C21H20O9 | 416.4 | ↓NFκβ, ↑AMPK, ↑ACC, ↑GSK-3β, ↓CREB | MCF-7/Adr cells | 20–100 µM | Bladder, liver, and lung cancer | [240] |
| Gardenia jasminoides Ellis | Genipin | C11H14O5 | 226.23 | ↓Bcl-2, ↑ Bax, ↑caspase-3, ↑JNK, ↑p38 MAPK | MDA-MB-231 cells | IC50: 327 µM | Gastric, multiple myeloma, and bladder cancer | [241] |
| Tetradium ruticarpum | Evodiamine | C19H17N3O | 303.4 | ↓u-PA, ↓MMP 9, Bcl-2, ↑ Bax, ↓CD1, ↓CDK6, G0/G1 arrest | MDA-MB-231 cells and xenograft BALB/c mice | IC50: 90 µM | Bladder, colorectal, pancreatic, and melanoma | [242] |
| Euphorbia prolifera Buch-Ham | Myrsinol diterpene (J196-10-1) | n.d. | n.d. | ↓MDR, ↓Pgp efflux, ↑ATP hydrolysis | MCF-7/Adr cells | 0.39–50 µM | Gastric, colon and lung cancer | [243] |
| Solanum nigrum L. | α-Solanine | C45H73NO15 | 868.1 | ↑Bax, ↓Bcl-2, ↓Ψm | 4T1 cells and xenograft BALB/c mice | IC50: 17 µM | Pancreatic, esophageal, and prostate cancer | [244] |
| Melonidus suaveolens | Melosuavine I | C41H42N4O6 | 686.79 | ↑caspase 3, ↓Bcl-2, ↑p53 | BT549 cells | IC50: 0.89 µM | Lung, colon, and prostate cancer | [245] |
| Cnidium monnieri (L.) Cusson. | Osthole | C15H16O3 | 244.28 | ↑caspase 9 and 3, ↓PARP, ↑p53 and p23, ↓Cdk2 and CD1 | MDA-MB 435 cells | 20–100 µmol/L | Liver and lung cancer | [246] |
| Ophiopogon japonicus | Ophiopogonin D | C44H70O16 | 855 | ↑Caspase 9 and 8, ↓cyclin B1 and G2/M arrest | MCF-7 cells | 12.5–50 µmol/L | Prostate and laryngeal carcinoma | [247] |
| Panax ginseng C. A. Mey. | Ginsenoside Rg5 | C42H70O12 | 767 | ↑p53, p21 and p15, ↓CD1, CE2 and Cdk4, ↑caspase 6,7,8 and 9, ↓PARP | MCF-7 cells | 25–100 µM | Leukemia, gastric, and cervical cancer | [248] |
| Epimedium brevicornum | Icariside II | C27H30O10 | 514.5 | ↑Cl-Caspase-9,8,7,3, ↑ Cl-PARP, ↑ Bax, ↑Bcl-xL, ↑BimL, ↑Fas, ↓ FasL, ↑FADD, ↓MMP, ↑Cyto- C, ↑AIF | MCF-7 and MDA-MB-231 cells | IC50: 72.73 and 97.14 µM | Lung, melanoma, epidermoid, and prostate cancer | [249] |
| Ovarian cancer | ||||||||
| Tripterygium wilfordii Hook | Triptolide | C20H24O6 | 360.4 | ↓ MMP7 and MMP19, ↑Ecadherin | SKOV3, A2780 cells and SKVO3 xenograft mice | 1.5–150 nM | Colon, renal, and cervical cancer | [250] |
| Panax ginseng | Ginsenoside 20(S)-Rg3 | C42H72O13 | 785 | ↑ Caspase-3 and 9, ↓ PI3K/Akt and IAP | HO-8910 cells | 25–100 µg/mL | Lung, prostate, and breast cancer | [251] |
| Syzygium aromaticum | Kumatakenin | C17H14O6 | 314.29 | ↑ Caspase-3,8 and 9, ↓MCP-1, ↓RANTES, ↓IL-10, ↓VEGF, ↓MMP 2 and 9 | SKOV3 and A2780 cells | <100 µg/mL | Breast, liver, colon, and gastric cancer | [252] |
| Pueraria mirifica | Daidzein | C15H10O4 | 254.24 | ↑Bcl-2, ↑cym-c, ↑cleaved caspase-3/9, G2/M cell arrest, ↓pCdc25c, ↓pCdc2, ↓cyclin B1, ↓MMP-2/9 | SKOV3 cell line | IC50: 20 µM | Breast, colon, bladder, and pancreatic cancer | [253] |
| Thelypteris torresiana (Gaud) | Protoapigenone | C15H10O6 | 286.24 | ↓p-Cdk2, ↓Cdk2, ↓p-Cyclin B1, ↓Cyclin B1, ↑p-Cdc25C, ↓Bcl-xL, ↓Bcl-2, ↑caspase-3 | MDAH-2774 and SKOV3 cells | IC50: 0.69 and 0.78 µM | Lung, breast, and prostate cancer | [254] |
| Epimedium brevicornum | Icariin | C33H40O15 | 676.7 | ↑Caspase-3, ↓miR-21, ↑PTEN and RECK, ↓Bcl-2 | A2780 cells | 13–100 µM | Esophageal, prostate, and liver cancer | [255] |
| Icaritin | C21H20O6 | 368.4 | ↑caspase 3- 9, ↑p53 and ↓Akt/mTOR pathway | OV2002, C13*, A2780cp and PDXs in NOD/SCID mice | 10–50 µM | Endometrial, bladder, colorectal, and prostate cancer | [256] | |
| Gundelia tournefortii | Stigmasterol | C29H48O | 412.7 | ↑ROS, ↑caspase 3 and 9, Bax, ↑BAk, ↑cym-c, ↓VEGFA, ↓MMP 2,9 and 14 | OV90 and ES2 cells | 5–20 µg/mL | Gastric, skin, liver, and lung cancer | [257] |
| Vitex Agnus-castus L. | Casticin | C19H18O8 | 374.3 | ↑FOXO3a, ↓FoxM1, ↓survivin, ↓PLK1, p27KLP1 | SKOV3 and A2780 cells | 2.5–10 µmol/L | Gastric, gallbladder, cervical, and melanoma | [258] |
| Carya cathayensis Sarg | Juglone | C10H6O3 | 174.15 | ↑ROS, ↑p21, ↑Bax, ↑Bad, ↑Cyto c, ↓CDK2, cdc25A, CHK1, and cyclin A, ↓Bcl-2 and Bcl-xL, ↓Cyclin A, S-phase cell cycle arrest | Ishikawa cells | IC50: 20.81 µM | Glioma, lung, and leukemia | [259] |
| Scutellaria baicalensis Georgi | Baicalin and Baicalein | C21H18O11, C15H10O5 | 446.4, 270.24 | ↓VEGF, HIF-1α, cMyc, and NFκB | OVCAR-3, IOSE-364, and CP-70 cells | 5–160 µM | Burkett lymphoma, colorectal, pancreatic, prostate, and osteosarcoma | [260] |
| Potamogeton crispus L. | Luteolin-3’-O-β-D-glucopyranoside | C21H20O11 | 448.4 | ↓MMP-2, ↓MMP-9, G1/S phase arrest | ES-2 cells | 15–240 µg/mL | Colon and breast cancer | [261] |
| Artemisia annua | artesunate | C19H28O8 | 384.4 | ↑ROS, ↑p21, ↓CDKs, ↓Rb, ↓E2F-1, ↓CDC25C, G2/M cell cycle arrest | HEY, IGROV-1, OVCAR8, and OVCAR3 cells, and ID8 xenograft in C57BL/6 mice | IC50: 0.51–31.89 µM | Leukemia, pancreatic, and breast cancer | [262] |
| Arctium lappa | Arctigenin | C21H24O6 | 372.4 | ↓STAT3, ↓survivin, ↓iNOS, ↑caspase3 | OVCAR3 and SKOV3 cells | IC50: 10 µM | Breast, colon, and lung cancer | [263] |
| Asparagus officinalis L. | Asparanin A | C39H64O13 | 740.9 | ↓Bak/Bcl-xl, ↑ROS, ↑Cyto c, ↓Δψm, ↑caspases, G0/G1 cell cycle arrest, ↓PI3K/Akt/mTOR | Ishikawa cells and xenograft BALB/c mice | IC50: 9.34 µM | Liver and pancreatic cancer | [264] |
| Salvia miltiorrhiza | Cryptotanshinone | C19H20O3 | 296.4 | ↑caspase3 and 9, ↑Bax/Bcl-2, ↓MMP 2 and 9 | A2780 cells | 5–30 µM | Leukemia, prostate, and colon cancer | [265] |
| Colon cancer | ||||||||
| Asiatic Moonseed | Dauricine | C38H44N2O6 | 624.8 | ↓cyclin D1, ↓COX2, ↓cMyc, ↓survivin, ↓Bcl-2, ↓IAP1, ↓MMP 9, ↓ICAM1, ↓VEGF, ↓NFκβ | HCT116, HCT8, SW480 and SW260 cells | 5–20 µM | Pancreatic and renal cell carcinoma | [266] |
| Withania somnifera | Withaferin A | C28H38O6 | 470.6 | ↑ROS, ↓ Bcl-2/Bax, ↑caspase3 and 9, ↓ΔΨm | RKO and HCT116 cells | 0.1–10 µM | Lung, breast, and pancreatic cancer | [267] |
| Piper nigrum | Piperine | C17H19NO3 | 285.34 | ↓wnt/β-catenin pathway | SW480 and SW480-pBAR/Renilla, HCT116, DLD1, RKO | 30–100 µM | Lung, liver, breast, and brain cancer | [268] |
| Crocus sativus | Crocetin | C20H24O4 | 328.4 | ↑p53, ↑PIDD, ↑Bax, ↑FAS, ↑caspase-3, -8 and -9 | HCT116 and HT29 cell lines | 100 µM | Prostate, breast, pancreatic, and gastric cancer | [269] |
| Cynanchum paniculatum | Antofine | C23H25NO3 | 363.4 | ↓proliferation, ↑cytotoxicity, G2/M cycle arrest | Col2 and A549 cells | IC50: <9 ng/mL | Breast, lung, and renal carcinoma | [270] |
| Zingiber zerumbet | Zerumbone | C15H22O | 218.33 | ↑ROS, ↓ Bcl-2/Bax, ↑caspase-3/-8/-9, ↓ΔΨm, G2/M cycle arrest | SW480 cell line | IC50: 102 µM | Oral, breast, lung, and prostate cancer | [271] |
| Hymenocallis littoralis | Pancratistatin | C14H15NO8 | 325.27 | ↑LC3II, ↑beclin-1, ↑Bax, ↓cyclin B1, ↓cdc25c, G2/M cycle arrest | SW948, DLD1, HTC15, and HT29 cells | IC50: 15–25 µM | Lymphoma, breast, liver, skin, and teratocarcinoma | [272] |
| Saussurea lappa | Costunolide | C15H20O2 | 232.32 | ↓Survivin, ↓β-catenin, ↓galectin-3, ↓cyclin D1, G2/M cycle arrest | SW480, L-Wnt3a cells | 0.5–5 µM | Breast, prostate, and ovarian cancer | [273] |
| Rehmannia glutinosa | Catalpol | C15H22O10 | 362.33 | ↓VEGF, ↓EGFR2, ↓HIF-1α, ↓IL-1β, ↓IL-6 and 8, ↓iNOS | CT26 cells and xenograft in mice | <40 µM | Gastric, lung, and liver cancer | [274] |
| Laminaria japonica | LJGP | n.d. | n.d. | ↓CDK2, ↓PCNA, ↓E2F-1, ↓cyclin E, ↓cyclin D1, ↓PARP, ↑p27, ↑caspase 9, ↓Bcl-2, G1 phase arrest | HT-29, HepG2 and AGS cells | IC50: 100 µg/mL | Lung, liver, and cervical carcinoma | [275] |
| Rosmarinus officinalis | Rosmarinic acid | C18H16O8 | 360.3 | ↑E-cadherin, ↓N-cadherin, ↓twist, ↓vimentin, ↓MMP 2 and 9, ↓ICAM-1, ↓ITGβ1 | CT26 and HCT116 cells | 50–200 µM | Breast, gastric, leukemia, and cervical cancer | [276] |
| Coptis chinensis | Berberine | C20H18NO4+ | 336.4 | ↑p21, ↓PARP, ↑caspase 8, ↓VEGF, ↓COX2, ↓Bcl-2, G2/M phase arrest | SW480 cells | 0.5–50 µM | Prostate, cervical, esophageal, thyroid, and gastric cancer | [277] |
| Sanguinaria canadensis | Sanguinarine | C20H14NO4+ | 332.3 | ↑ROS, ↓MMP, ↑caspase-3, 8 and 9, ↓Bcl-2, ↓XIAP, ↑Egr-1 | HCT-116 cell line | 0.3–1.2 µM | Breast, prostate, cervical, and pancreatic cancer | [278] |
| Nauclea orientalis | Naucleaoral A and B | C20H20N2O3 | 336.38 | ↑cytotoxicity | Hela and KB cells | IC50: 4.0 and 7.8 µg/mL | Cervical, bladder, and pancreatic cancer | [279] |
| Glycyrrhiza uralensis | Licoricidin | C26H32O5 | 424.5 | ↑caspase-3, 8 and 9, ↓CDK1, ↑AMPK, ↓Akt/mTOR, G1/S phase arrest | SW480 cells and xenograft BALB/c mice | IC50: 7.2 µM | Gastric, lung, prostate, and osteosarcoma | [280] |
| Carpobrotus edulis | Rutin | C27H30O16 | 610.5 | ↑caspase 3, G0/G1 phase arrest | HCT116 and HaCaT cells | 1000 µM IC50: 679 µM | Breast, lung, and cervical cancer | [281] |
| Scutellaria baicalensis | Baicalin and Baicalein | C21H18O11, C15H10O5 | 446.4, 270.24 | ↓hTERT, ↓MAPK, ↓ERK, ↑p38 | HT-29, SW480 cells and HCT-116 xenograft NSG mice | 10–150 µM | Breast, prostate, and pancreatic cancer | [282] |
| Nicotiana glauca | Scopoletin | C10H8O4 | 192.17 | ↓ERK1, ↓VEGF-A, ↓FGF-2 | HUVEC, CCD18-Co and HCT116 Xenograft mice | IC50: 0.06 µM | Breast, lung, and skin cancer | [283] |
| Morus australis | Morusin | C25H24O6 | 420.5 | ↓ PI3K/Akt, ↓PDK1, ↓XIAP, ↓cMyc, ↓NFκβ, ↑caspase-3, 8 and 9, G1 phase arrest | HT-29 cells | IC50: 6.1–12.7 µM | Breast, ovarian, and prostate cancer | [284] |
| Allium sativum | Diallyl disulphide | C6H10S2 | 146.3 | ↓GSK3β, ↓NFκβ | SW480 cells and AOM/DSS mouse model | 2.5–40 µM | Lung, breast, and gastric cancer | [285] |
| Nandina domestica | Protopine | C20H19NO5 | 353.4 | ↑p53, ↑p21, ↑Bax, ↑caspase-3 and 7, ↑LC3-II | HCT116 cells | 10–40 µM | Prostate, breast, ovarian, and head and neck cancer | [286] |
| Brain cancer | ||||||||
| Nigella sativa | Thymoquinone | C10H12O2 | 164.2 | ↑LC3-II, ↑p62, ↓MMP 2 and 9, ↓FAC, ↓Nf-kβ, ↓ERK, ↓Akt, ↓mTOR | T98MG and U87MG cells | 10–40 µM IC50: 10.3 µM and 8.3 µM | Breast, liver, colon, and lung cancer | [287,288] |
| Radix Angelica sinensis | Z-ligustilide | C12H14O2 | 190.24 | ↓RhoA, ↓Cdc42, ↓Rac1 | T98MG cells | 2.5–25 µM | Breast, prostate, and colon cancer | [289] |
| Panax ginseng C. A. Mey | Ginsenoside Rh2 | C36H62O8 | 622.9 | ↑miR128, ↑E2F3a, ↑caspase-3 | T98MG, A172, and U251 cells | 12 µg/mL | Breast, ovarian, colon, and prostate cancer | [290] |
| Bolbostemma paniculatum | Tubeimoside-1 | C65H102O29 | 1347.5 | ↓Bcl-2, ↑ Bax, ↑caspase-3, ↑Cyto- C, ↑ROS | U251 and U87 cells | 10–50 µg/mL | Gastric, liver, ovarian, and lung cancer | [291] |
| Thuja occidentalis | α-/β-Thujone | C10H16O | 152.23 | ↓VEGF, ↓Ang-4, ↓CD31 | U87-MG and C6 cells xenograft in mice | LD50: 400 and 300 µg/mL | Melanoma, breast, lung, and colon cancer | [292] |
| Rubia cordifolia L. | Mollugin | C17H16O4 | 284.31 | ↓ Akt, ↓ P70S6K, ↓ mTOR, ↓ ERK ½, ↑JNK, ↑p38 | U87MG, U251 and MKN45 cells | 10–40 µM | Breast, colon, ovarian, and lung cancer | [293] |
| Garcinia brasiliensis | 7-epiclusianone | C33H42O4 | 502.7 | ↓cyclin A, ↑caspase-3, S and G2/M phase arrest | U251MG and U138MG cell lines | IC50: 23 and 18.52 µM | Colon, melanoma, breast, lung, and ovarian cancer | [294] |
| Escherichia coli | Selenocysteine | C3H6NO2Se | 167.06 | ↑ROS, ↑p21waf1/cip1, ↑p53, ↓Akt, ↑p38MAPK, ↑JNK, ↑ERK, S phase cycle arrest | U251 and U87 cell lines | 5–20 µM | Breast, lung, and prostate cancer | [295] |
| Anula helenium | Alantolactone | C15H20O2 | 232.32 | ↑ROS, ↓GSH, ↓Bcl-2, ↑ Bax, ↑p53, ↑Cyto- C, ↑caspase-3/-9, ↓ΔΨm, ↓Nf-kβ | U87, U373 and LN229 cells | IC50: 33–36 µM | Lung, breast, liver, and pancreatic cancer | [296] |
| Carpesium nepalense | Nepalolide A | C20H28O6 | 364.4 | ↓IkB-α, ↓IkB-β, ↓iNOS, ↓NF-kβ | C6 cell line | 2–10 µM | Brain cancer | [297] |
| Buxus microphylla | Cyclovirobuxine D | C26H46N2O | 402.7 | ↑ Bax, ↓Bcl-2, ↑caspase-3, S and G0/G1 phase arrest | T98G and Hs683 cell lines | 15–240 µmol/L | Gastric, colon, breast, and prostate cancer | [298] |
| Euscaphis japonica | Pomolic acid | C30H48O4 | 472.7 | ↑caspase-3 and 9, ↑ROS, ↓MRP1 | A172, GBM-1 and U87 cells | IC50: 8.82, 9.72 and 11.09 µg/mL | Leukemia, melanoma, gastric and uterine cancer | [299] |
| Danshen | Salvianolic Acid B | C36H30O16 | 718.6 | ↑ROS, ↑p53, ↑p38 MAPK | U87 cell line | 1–100 µM | HNSCC, breast, and colon cancer | [300] |
| Glycine max | Soyasapogenol B | C30H50O3 | 458.7 | ↓STAT3, ↓M2 polarization, ↑M1, ↑IL-12 | U373-MG, SaOS2, and LM8 cells xenograft in mice | 1–100 µM | Gastric, breast, colon, and renal cancer | [301] |
| Rosmarinus officinalis | Carnosol | C20H26O4 | 330.4 | ↑p53, ↓twist, ↓Zeb1, ↓slug, ↑miR-200c | U87MG, T98G, U373 MG | IC50: <40 µM | Lymphoma, lung osteosarcoma, and gastric cancer | [302,303] |
| Tillandsia recurvata | HLBT-100 | C19H20O8 | 376.4 | ↑caspase-3, and 7, G1 cell cycle arrest, ↓angiogenesis | NCI60 cell lines (U87-cells) | GI50 values: <0.100 µM IC50 for U87: 0.054 µM | Breast, prostate, leukemia, and melanoma | [304] |
| Ligusticum chuanxiong hort | Tetramethylpyrazine | C8H12N2 | 136.19 | ↓CXCR4 | C6 cell line | 100 µM | Breast, liver, colon, and lung cancer | [305] |
| Garcinia hanburyi Hook. f | Gambogenic acid | C38H46O8 | 630.8 | ↓cyclin E, ↓cyclin D1, ↓EGFR, ↓Akt, ↓GSK3β, Go/G1 phase arrest | U251 cell line | 0.75–6 µM | Gastric, breast, and lung cancer | [306] |
| Vitis vinifera | Resveratrol | C14H12O3 | 228.24 | ↓Akt, ↑p53 | U87 and patient derived (22,33 and 44 GSC) cells | 5–100 µM | Colon, gastric, and breast cancer | [307] |
| Curcuma aromatica Salisb. | Germacrone | C15H22O | 218.33 | ↑p53, ↑ Bax, ↓Bcl-2, ↑p21, ↓CD1, ↓CDK2, G1 phase arrest | U87 and U251 cells | 50–250 µmol/L | Breast, prostate, and liver cancer | [308] |
| Acori Graminei Rhizoma | Volatile Oil (VOA) | n.d. | n.d. | ↑caspase-3, 8 and 9, ↑ Bax/Bcl-2, ↑LC3-II/I, ↑atg5, ↑beclin1, ↓p62 | U87, U251, 3T3 and A172 cells | 25–250 µg/mL | Pancreatic and breast cancer | [309] |
| Trichosanthes kirilowii Maxim. | Trichosanthin | 247 Amino acids | 27 kDa | ↓LGR5, ↓β-catenin, ↓pGSK-3βSer9, ↓cMyc, ↓CD1 | U87 and U251 cells | IC50: 40 and 51.6 µM | Leukemia and cervical cancer | [310] |
| Streptomyces staurosporeus | Staurosporine | C28H26N4O3 | 466.5 | ↑caspase-3, ↓TDP-43 | U87 cell line | IC50: 5µM | Lung, breast, prostate, and colon cancer | [311] |
| Dysosma versipellis | Deoxypodophyllotoxin | C22H22O7 | 398.4 | ↓Cdc2, ↓CB1, ↓Cdc25C, ↑caspase-8 and 9, ↓Bcl-2, ↓Bcl-xL, G2/M phase arrest | U-87 MG and SF126 cells | IC50: 15.06 and 13.95 nM | Lung, breast, prostate, and colon cancer | [312] |
| Anemone taipaiensis | Saponin B | C48H78O17 | 927.1 | ↑Fas-l, ↑caspase-3, ↓Bcl-2, G1/S phase arrest | U87MG cells | IC50: 6.7 µmol/L | Leukemia and breast cancer | [313] |
| Liver cancer | ||||||||
| Strychnos nux-vomica Linn | Brucine | C23H26N2O4 | 394.5 | ↓HIF-1, ↓MMP-2, ↓FN, ↓LOX, ↓CD | SMMC-7721, HepG2, and HCC in Male Kunming mice | 20–150 µM | Colon and breast cancer | [314] |
| Azadirachta indica | Nimbolide | C27H30O7 | 466.5 | ↑caspase-3,7 and 9, ↑ Bax, ↓Bcl-2, ↓Mcl-1, ↓XIAP, ↓ c-IAP1, ↓c-IAP2, G2/M phase arrest | PLC/PRF/5 and Huh-7 cells xenograft Balb/c mice | 1–5 µM | Pancreatic, breast, lung, and colon cancer | [315] |
| Gardenia jasminoides ellis | Geniposide | C17H24O10 | 388.4 | ↓miR-224, ↓wnt/βcatenin, ↓Akt | HepG2 and Huh7 cells | 100–500 µM | Brain, oral, skin, and colon cancer | [316] |
| Solanum nigrum L. | Solamargine | C45H73NO15 | 868.1 | ↓pcna, ↓Ki67↓Bcl-2, ↑ Bax, ↑caspase-3 and 9, G2/M phase arrest | SMMC-7721 and HepG2 cells | IC50: 12.17 and 20 µM | Colon, lung, prostate, and breast cancer | [317] |
| Matricaria recutita | α- bisabolol | C15H26O | 222.37 | ↑caspase-3,-8 and -9, ↑Fas, ↓Bcl-2, ↑p53, ↑Nf-kβ, | HepG2, ECa109, PC-3 and Hela cells | 1–20 µM | Colon, brain, endometrial, and prostate cancer | [318] |
| Ganoderma lucidum | Ganoderic acid A | C30H44O7 | 516.7 | ↓cyclin D1, ↑p21, ↑cleaved caspase 3, G0/G1 phase arrest | HepG2 and SMMC-7721 cells | IC50: 187.6 and 158.9 µmol/L | Prostate, breast, lung, and meningioma | [319] |
| Thalictrum glandulosissimum | Hernandezine | C39H44N2O7 | 652.8 | ↑AMPK, ↑Atg-7 | HepG2 and Hep3B cells | IC50: 7.42 and 6.71 µM | Lung, prostate, breast, and cervical cancer | [320] |
| Angelica gigas Nakai | Decursin | C19H20O5 | 328.4 | ↑LATS1, ↑βTRCP, ↑p-YAP, ↑cleaved caspase 3, ↑cleaved PARP, G1 phase cell cycle arrest | HepG2, Huh-7 cells and tumor xenograft in mice | 5–80 µM | Gastric, lung, prostate, and lymphoma | [321] |
| Lavandula officinalis | Linalool | C10H18O | 154.25 | ↓cyclin A, ↓CDK4, ↑p21, ↑p27, ↑ROS, ↑caspase-3, ↓Ras, ↓Akt, ↓mTOR, G0/G1 phase arrest | HepG2 cell line | 0–2.5 mM | Leukemia, breast, prostate, and ovarian cancer | [322] |
| Tylophora indica | Tylophorine | C24H27NO4 | 393.5 | ↓cyclin A2, G1 phase cell cycle arrest | HepG2, HONE-1, and NUGC-3 cells | 2 µM | Breast, stomach, nasopharyngeal, and colon cancer | [323] |
| Patrinia scabra Bunge | Lariciresinol | C20H24O6 | 360.4 | ↓ΔΨm, ↑Cyto- C, ↑caspase-3 and 9, ↑PARP, ↓Bcl-2/Bax | HepG2 cells | IC50: 208 µg/mL | Leukemia, breast, and prostate cancer | [324] |
| Oroxylum indicum | Oroxin B | C27H30O15 | 594.5 | ↑PTEN, ↓COX-2, ↓VEGF, ↓p-Akt, ↓PI3K | SMMC-7721 | 0.34–1.68 µM | Breast, lung, and lymphoma | [325] |
| Stephania tetrandra | Tetrandrine | C38H42N2O6 | 622.7 | ↓wnt/β-catenin, ↓MTA1, ↑E-cadherin, ↑occludin, ↓Vimentin | Huh7, Hep3B and HCCLM9 xenograft Balb/c mice | 0.5–4 µM | Colon, esophageal, and pancreatic cancer | [326] |
| Scutellaria lateriflora | Scutellarein | C15H10O6 | 286.24 | ↓HIF-1α, ↓Flt-1, ↓VEGFA, ↓MMP 2 and 9, ↑caspase-3 | HepG2, MCF-7, EAC, A549 and liver carcinoma and ascites lymphoma model in mice | IC50: <13.8 µM | Gastric, colon, lung, and fibrosarcoma | [327] |
| Toddalia asiatica Lam | Chelerythrine | C21H18NO4+ | 348.4 | ↓MMP 2 and 9, ↓p-FAK ↓PI3K, ↓Akt, ↓mTOR, ↓c-JNK, ↓ERK | Hep3B Cell line | 0.625–5 µM | Breast, prostate, renal, and lung cancer | [328] |
| Astragalus complanatus R.Br. | (FAC) flavonoids | n.d. | n.d. | ↑caspase-3 and 8, ↑ Bax, ↑p21, ↑p27, ↓CDK1, CDK4, ↓cyclin B1, ↓cyclin D1, G0/G1 and S phase arrest | SMMC-7721 and HepG2 cells | IC50: 48 and 53 µg/mL | Breast and nasopharyngeal | [329] |
| Quercus Iberica | Quercetin | C15H10O7 | 302.23 | ↑E-cadherin, ↓MMP9, ↑LC3, ↓Vimentin, ↓Jak2, ↓STAT3, | LM3 cells and xenograft mice model | 20–200 µM | Breast, colon, ovarian, and pancreatic cancer | [330] |
| Trichosanthis Radix | Cucurbitacin B | C32H46O8 | 558.7 | ↓cdc2, ↓cyclin D1, ↓c-Raf, S phase arrest | BEL-7402 cells and xenograft mice | IC50: 0.32 µM | Brest, pancreatic, and laryngeal cancer | [331] |
| Colchium speciosum | Colchicine | C22H25NO6 | 399.4 | ↑AKAP12, ↑TGFB2, ↑MX1, ↓APOH, ↑GDF15, ↑IL32 | HCC24 and HCC38/KMUH, F28 and F59/KMUH cell lines and Balb/c-nu xenograft | 2 and 6 ng/mL | Thyroid, oropharyngeal, and breast cancer | [332] |
| Head and Neck cancer | ||||||||
| Wilkstroemia elliptica Merr. | Umbelliferone | C9H6O3 | 162.14 | ↑ROS, ↓MMP, G0/G1 cycle arrest | HOC KB cells | IC50: 200 µM | Renal prostate, lung, and breast cancer | [333] |
| Dioscorea nipponica | Dioscin | C45H72O16 | 869 | ↑p53, ↓Cyclin A, ↓CDK2, ↓p-ERK, ↓Bcl-2, ↑p-JNK, ↑p-p38, ↑Bax, ↑cleaved caspase-3/-9 | NP69, Hep-2 and TU-212 cells | IC50: <5 µg/mL | Gastric, breast, lung, ovarian, and colorectal cancer | [334] |
| Maclura pomifera | Osajin | C25H24O5 | 404.5 | ↑Bax, ↓Bcl-2, ↑Fasl, ↑cym-c, ↓GRP78, ↑caspase-3,4,8 and -9 | TW076, TW04 and CG-1 cells | IC50: 5 µM | Kidney, prostate, breast, and colon cancer | [335] |
| Cichorium intybus | Esculetin | C9H6O4 | 178.14 | ↓pJAK1/2, ↑ROS, ↓STAT3, G1/S cycle arrest | TU-212, M4e, Hep-2 xenograft in mice | IC50: 2.969, 12.88 and 1.958 µM | Pancreatic, prostate, colon, and lung cancer | [336] |
| Serratia marcescens | Prodigiosin | C20H25N3O | 323.4 | ↓Cyclin D1, ↑beclin-1, ↓mTOR, ↓PI3K/Akt, G0/G1 phase arrest | OECM1 and SAS cell lines | IC50: 1.59 and 3.25 µM | Breast, gastric, colon, and hematopoietic cancer | [337] |
| Albatrellus confluens | Neoalbaconol | C22H34O3 | 346.5 | ↓PDK1, ↓PI3K/AKT HK-2, ↑RIP1, ↑RIP3 | NP69, k562,MCF-7 and A549 and C666-1 xenograft in mice | IC50: ≤18 µM | Lung, breast, colon, and gastric cancer | [338] |
| Haematococcus pluvilis | Astaxanthin | C40H52O4 | 596.8 | ↓PI3k/Akt, ↓STAT3, ↓Nf-kβ, ↓miR-21, ↓HOTAIR | SCC131 and SCC4 cells | IC50: 720 and 700 µM | Pancreatic, breast, colon, and melanoma | [339] |
| Geranium thunbergii | Geraniin | C41H28O27 | 952.6 | ↓MMP2, ↓Fak, ↓Src, ↓ERK ½ | SCC-9 and SCC-14 cells | 20–80 µM | Brain, breast, colon, ovarian, bladder, and osteosarcoma | [340] |
| Forsythia suspensa | Phillygenin | C21H24O6 | 372.4 | ↑Caspase-3/-9, ↑Bax, ↑ROS, ↓Bcl-2, G2/M phase arrest, ↓Nf-kβ | SH-1-V1 cell line and xenograft in mice | IC50: 6 µM | Lung, liver, and pancreatic cancer | [341] |
| Enicosanthellum phalcrum | Liriodenine | C17H9NO3 | 275.26 | ↑Bax, ↑Caspase-3, ↓Bcl-2, G2/M phase arrest | ECA-109 cell line | 0.1–20 µM | Breast, prostate, and gastric cancer | [342] |
| Halichondria sp. | Ilimaquinone | C22H30O4 | 358.5 | ↑ROS, ↑LC3B-II, ↑Atg5, ↓p-pAkt, ↓p-p38, ↓HIF-1α, ↓Mcl-1, ↓Bcl-2 | SCC4 and SCC2095 cells | IC50: <9 µM | Prostate, lung, and colon cancer | [343] |
| Tabernaemontana catharinensis | Coronaridine | C21H26N2O2 | 338.4 | ↑apoptosis, ↑cytotoxicity | Hep-2 cell line | IC50: 54.47 µg/mL | Leukemia, breast, colon, and gastric cancer | [344] |
| Chelidonium majus | Ukrain | C66H75N6O18PS | 1303.4 | ↓EGFR, ↓AKT2, ↓STAT3, JAK1, ↓β-catenin, ↑CYP1A1, ↑CYP1B1 | FaDu, HlaC78 cells | EC50: <11 µg/mL | Lung and prostate cancer | [345] |
| Prostate Cancer | ||||||||
| Cruciferae | Indole-3-carbinol | C9H9NO | 147.17 | ↓CDK6, ↑Bax, ↓Bcl-2, ↑p21, ↑p27, G1 phase arrest | PC-3 cells | 30–100 µM | Melanoma, colon, breast, and endometrial | [346] |
| Genista tinctoria | Genistein | C15H10O5 | 270.24 | ↓HOTAIR, miR-34a↓NFκβ, ↓Akt | PC3 and Du145 cells | 25 µM | Lung and breast cancer | [347,348] |
| Punica granatum | Ellagic acid | C14H6O8 | 302.19 | ↑IL-6, ↓STAT3, ↓Akt, ↓ERK | LNCaP and PC-3 cells | 20–100 µM | Breast and ovarian cancer | [349,350] |
| Stephania tetrandra | Fangchinoline | C37H40N2O6 | 608.7 | ↓NR4A1, ↓survivin, ↑ROS, ↑caspase 3 and 8 | MiaPaCa-2 and Panc-1 cells | IC50: 11.1 and 17 µM | Gastric, bladder, breast, and colon cancer | [351] |
| Melodinus khasianus | Khasuanine A | C21H24N2O3 | 352.43 | ↑Caspase-3, ↑p53, ↓Bcl-2 | PC3 cell line | IC50: 0.45 µM | Breast, lung, and colon cancer | [352] |
| Camptotheca acuminate | Camptothecin | C20H16N2O4 | 348.4 | ↑ROS, ↑c-Myc, ↑sp1, ↑PI3k/Akt, ↑hTERT, G2/M cycle arrest | LNCaP cells | 1–5 µM | Leukemia, breast, colon, and lung cancer | [353] |
| Andrographis paniculata | Andrographolide | C20H30O5 | 350.4 | ↓MMP11, ↑γH2AX, ↑ Caspase-3, and 7, G2/M phase arrest | PC3, LNCaP, and 22RV1 SCID orthotopic model | GI50: 26.2, 28.1 and 24.2 µM | Glioblastoma, renal, colon, and ovarian cancer | [354] |
| Dioscorea nipponica | Diosgenin | C27H42O3 | 414.6 | ↓Bcl-2, ↓beclin-1, ↑caspase-9, ↓ PI3K, ↓Akt, ↓mTOR, | DU145 cell line | IC50: 6.757 µg/mL | Breast, liver, and gastric cancer | [355] |
| Murraya koenigii | Mahanine | C23H25NO2 | 347.4 | ↓DNMT1, ↓DNMT3B, ↓PDK1, ↓Akt, ↑RASSF1A | LNCaP and PC-3 cells | 10–20 µM | Colon, brain, and lung cancer | [356] |
| Quercus petraea | Procyanidin | C30H26O13 | 594.5 | ↓ΔΨm, ↑ apoptosis, and necrosis | PC-3 cell line | 100–300 µg/mL | Breast, lung, stomach, and colon cancer | [357] |
| Santalum album | α-santalol | C15H24O | 220.35 | ↓Survivin, ↓p-Akt, ↑ Caspase-3, ↑cleaved PARP | LNCaP and PC-3 cells | 20 and 40 µM | Breast, colon, and skin cancer | [358] |
| Goniothalamus spp. | Altholactone | C13H12O4 | 232.23 | ↓p65, ↓NF-kβ, ↓STAT3, ↓survivin, ↓Bcl-2, ↑Bax, S phase arrest | DU145, PC3, and LNCap cells | 20 and 40 µM IC50: 38.5 µM | Colon, leukemia, and bladder cancer | [359] |
| Nauclea subdita | Subditine | C20H15N3O2 | 330.1237 | ↑ROS, ↓MMP, ↑cym-c, ↑caspase-3,7 and 9, ↓Bcl-2 | LNCaP and PC-3 cells | IC50: <14 µM | Breast, lung, and colon cancer | [360] |
| Isodon eriocalyx | Eriocalyxin B | C20H24O5 | 344.4 | ↑cleaved caspase 3 and 8, ↑cleaved PARP, ↑LC3B-II, ↓Akt/mTOR | PC3 and 22RV1 cells | IC50: <4 µM | Breast, colon, lung, and bladder cancer | [361] |
| Garcinia indica | Garcinol | C38H50O6 | 602.8 | ↑ Bax/Bcl2, ↑caspase-3 and 9, ↓PARP, ↑PI3K, ↑Akt, ↑mTOR | PC-3 cells and xenograft mice | 30 µM | Breast, lung, leukemia, and bladder cancer | [362] |
| Allium atroviolaceum | Tricin | C17H14O7 | 330.29 | ↓MiR-21 | PC-3 cell line | IC50: 117.5 µM | Breast, colon, and liver cancer | [363] |
| Sinomenium acutum | Sinomenine | C19H23NO4 | 329.4 | ↓miR-23a, ↓CD1, ↓CDk4, ↓Bcl-2, ↓MMP-2 and 9, ↓PI3K/Akt, ↓JAK/STAT | PC-3 cells | 0.25–1 mM | Gastric, ovarian, breast, and lung cancer | [364] |
| Hematological Cancer | ||||||||
| Artemisia annua | Dihydroartemisinin | C15H24O5 | 284.35 | ↓VEGF, ↓ERK1/2 | RPMI18226 MM cells | IC50: 30.24 µmol/L | Brain, melanoma, and ovarian | [365] |
| Artemisia absinthum | Cardamonin | C16H14O4 | 270.28 | ↑ROS, ↑Ca2+, ↑ Caspase-3, -8 and-9, ↓Bcl-2, ↑Bax, ↑ cyto-c, ↑AIF, ↑GRP78, ↑FasL, ↑DAP, | WEHI-3 cell line | 2–10 µM | Gastric, nasopharyngeal, prostate, and colon cancer | [366] |
| Myristica fragrans | Myristicin | C11H12O3 | 192.21 | ↑Caspase-3, ↑Cyto- C, ↓PARP, ↓ERCC1, ↓RAD50, 51, ↓ATM | K562 cells | IC50: 368 µM | Stomach, lung, and ovarian cancer | [367] |
| Ganggui luhui wan | Meisoindigo | C17H12N2O2 | 276.29 | ↑Caspase-3,8 and 9, ↑Bax, ↑Cyto- C, ↑PARP, ↓Bcl-2, | HL60 cell line | 20 µmol/L | Colon, breast, and lung cancer | [368] |
| Cananga odorata | Sampangine | C15H8N2O | 232.24 | ↑ROS, ↓ΔΨm, ↑Caspase-3, G1 phase arrest | HL60 cell line | 1–20 µM | Lung, head, and neck cancer | [369] |
| Ambrosia maritoma | Damsin | C15H20O3 | 248.32 | ↓c-Src, ↓AKT, STAT5, ↓NFkβ | CEM/ADR5000, CCRF CEM cells | IC50: 4.8 µM | Colon, breast, and lung cancer | [370] |
| Forsythia suspensa | Pinoresinol | C20H22O6 | 358.4 | ↑p21 WAF1/Cip1, G0/G1 phase arrest | HL60, HL60R, and K562 cells | IC50: 8 and 32 µM | Breast, prostate, and colon cancer | [371] |
| Danggui longhui wan | Indirubins | C16H10N2O2 | 262.26 | ↓c-Src, ↓Abl kinase, ↓Gsk3β | KCL-22 and T315I mutant KCL-22 cells | IC50: 0.3–0.9 µM | Colon, breast, renal, and pancreatic cancer | [372] |
| Arnica chamissonis | Helenalin | C15H18O4 | 262.3 | ↓NFkβ, ↑ROS | Jurkat T (J16) and Neo jurkat or Bcl-2 jurkat cells | 2–20 µM | Breast, prostate, and colon cancer | [373] |
| Cinnamosma fragrans | Capsicodendrin | C34H48O10 | 616.7 | DNA damage and proapoptotic | K562 and HL-60 cells | IC50: <0.5 µM | Breast, lung, and colon cancer | [374] |
| Cephalotaxus fortunei | Homoharringtonine | C29H39NO9 | 545.6 | ↓SP1/TET1/5hmC/FLT3/MYC | MA9.3ITD, MA9.3RAS, MONOMAC 6, Kasumi-1 and xenograft in mice | IC50: 9.2–36.7 nM | Colon, breast, and gastric cancer | [375] |
| Papaver somniferum | Noscapine | C22H23NO7 | 413.4 | ↓Bcl-2, ↓XIAP, ↓Mcl-1, ↓Bcl-xl, ↑Bax, ↓TRAF1, ↓IKK, ↓NFkβ, | KBM-5 and U266 cells | IC50: 84.4 and 155 µmol/L | Colon, breast, lung, and ovarian cancer | [376] |
| Streptomyces cinnamonensis | Monensin | C36H62O11 | 670.9 | ↓MyB, ↓MyB-NFIB | HEK-Luc, HEK-MyB-Luc, and MyB-NFIB ACC cells | 0.3–3 µM | Prostate, ovarian, colon, and pancreatic cancer | [377] |
| Brucea antidysenteriea | Bruceantin | C28H36O11 | 548.6 | ↑NOTCH1, ↑HES1, G1 phase arrest | MM-CSCs cells | IC50: 77 nM | Breast, colon, osteosarcoma, and lung cancer | [378] |
| Aglaia foveolata | Silvestrol | C34H38O13 | 654.7 | ↓FLT3, ↓miR155, ↓ p65 | Mv4-11, THP-1, AML blasts from patients and Mv4-11 xenograft murine model | IC50: 2.7, 3.8 and 12 nM | Liver, colon, and melanoma | [379] |
| Miscellaneous Cancer | ||||||||
| Cynanchum otophyllum | Paeoniflorin | C23H28O11 | 480.5 | ↑HTRA3, ↑Bax | Capan-1 and MIAPaCa-2 cells | IC50: 862.7, and 489.5 µM | Pancreatic, glioma, lung, and colon cancer | [380] |
| Periploca sepium bunge | Baohuoside-I | C27H30O10 | 514.5 | ↓β-catenin, ↓survivin, ↓cyclin D1 | Eca109 cells and Eca109-Luc xenograft in mice | 12.5–50 µg/mL IC50:24.8 µg/mL | Esophageal, breast, and leukemia | [381] |
| Ochrosia elliptica | Ellipticine | C17H14N2 | 246.31 | ↑AIF, ↑Cyto- C, ↑ROS, ↑caspase-3, 7 and 9, ↑ERK, ↑JNK, G2/M cycle arrest | RL95-2 cells | 1–10 µM | Endometrial, ovarian, thyroid, and breast cancer | [382] |
| Ilex hainanensis | Ilexgenin A | C30H46O6 | 502.7 | ↓IL-6, ↑TNF-αG0/G1 cycle arrest | RAW 264.7 and B16-F10 xenograft in mice | IC50: 66.57 and 27.34 µM | Melanoma, colon, breast, and cervical cancer | [383] |
| Dysoxylum binectariferum | Flavopiridol | C21H20ClNO5 | 401.8 | ↑caspase-3, 8 and 9, ↓Mcl-1 ↑cyclin-B1, G2/M cycle arrest | KKU-055, -100, -214 cells and KKU-213 xenograft in Balb/c mice | IC50: 40–213 nM | Osteosarcoma, leukemia, lung, and breast cancer | [384] |
| Crinum asiaticum | Crinamine | C17H19NO4 | 301.34 | ↓Snail1, ↓vimentin, ↓VEGF-A, ↓CDK4, ↓RHOA, ↓PLK1, ↓BCL2L1, ↓Akt1 | SiHa and C33a cell lines | IC50: 23.52 and 60.89 µM | Cervical, prostate, gastric, and colon cancer | [385] |
| Alpinia katsumadai | Alpinetin | C16H14O4 | 270.28 | ↓Bcl-2, ↓Bcl-xL, ↑ Bax, ↑Cyto- C, ↑XIAP, ↑caspase-3, 8 and 9 | BxPC-3, PANC1, and AsPC-1 cells | 20–80 µg/mL | Hepatoma, leukemia, colon, lung, and breast cancer | [386] |
| Combretum caffrum | Combretastatin A4 | C18H20O5 | 316.3 | ↓N-cadherin, ↓Vimentin, ↓slug, ↓snail1, ↓zeb1, ↓p-PI3K, ↓p-Akt | TPC1 cell line | 5 and 10 µM | Thyroid, breast, colon, and lung cancer | [387] |
| Heliotropium Indicum Linn | Indicine N-oxide | C15H25NO6 | 315.36 | ↑p53, cleave PARP, cleave DNA | Hela, MCF-7, PC3, and SiHa cell lines | IC50: 46–91 µM | Colon, breast, prostate, and head and oral cancer | [388] |
| Arnebia euchroma | Alkannin | C16H16O5 | 288.29 | ↑ROS, ↓ΔΨm, ↑p38 MAPK, ↑JNK | MDA-MB-231, HCT116, A549, Huh7, HepG2, MCF cells | IC50: 5–12 µM | Breast, colon, lung, liver, and glioma | [389] |
| Artemisia princeps | Jaceosidin | C17H14O7 | 330.29 | ↑pCdc25C, ↑p21, ↑ROS, ↓ERk ½, ↑ATM-Chk1/2, G2/M phase arrest | HES, HESC, Hec1, and KLE cell line | IC50: 52.68–147.14 µM | Endometrial, ovarian, glioblastoma, and oral cancer | [390] |
| Coccinia grandis | Kaempferol | C15H10O6 | 286.24 | ↑LC3-II, ↓p62, ↓G9a, ↑IRE1-JNK-CHOP | AGS, NCI-N87, MKN-74, SNU-216, and SNU-638 | IC50: 50 µM | Gastric, colon, breast, prostate, and pancreatic cancer | [391] |
| Daphne odora | Daphnetin | C9H6O4 | 178.14 | ↓RhoA, ↓Cdc42 | LM8 OS cells | 1–30 µM | Osteosarcoma, breast, lung, and colon cancer | [392] |
| Cerbera odollam | Cerberin | C32H48O9 | 576.7 | ↓cMyc, ↑Caspase 3 and 7, ↑ROS ↓Bcl-2, ↓Mcl-1, ↓STAT-3, ↓PLK-1, G2/M phase arrest | PANC-1, MIA Paca2, A549, HepG2, HT29 cells and mice model | GI50: <90 nM | Pancreatic, liver, colon, and breast cancer | [393] |
| Asiatic toad | Cinobufagin | C26H34O6 | 442.5 | ↓Bcl-2, ↓CB1, ↑p21, ↓CDC2, ↑puma, ↑caspase 3, G2/M phase arrest | EC-109, Kyse-150, and Kyse-520 cells | IC50: 0.91, 0.66 and 0.62 µM | Gastric, liver, colon, and melanoma | [394] |
| Astragalus membranaceus | Swainsonine | C8H15NO3 | 173.21 | ↑E-cadherin,↓N-cadherin, ↓Vimentin, ↓slug, ↓snail1, ↓zeb1, ↓p-PI3K, ↓p-Akt, ↓Twist1 | EC9706 and 293T cells | 10–100 µg/mL | Esophageal, colon, liver, and glioma | [395] |
| Magnolia officinalis | Honokiol | C18H18O2 | 266.3 | ↓cyclin D1, ↓CDK2 and 4, ↓Akt/mTOR, ↓p38, ↓ERK, G0/G1 phase arrest | WRO, SW579 cells, and ARO cells xenograft in Balb/c mice | IC50: 37.7, 19.9, and 36.3 µM | Thyroid, leukemia, prostate, and colon cancer | [396] |
| Polymethoxyflavone | Tangeritin | C20H20O7 | 372.4 | ↓ MMP, ↑ Caspase-3, -8 and -9, ↑ Bax, ↑ Bid, ↑ tBid, ↑ p53, ↑ p21/cip1, ↑ Fas and ↑ FasL | AGS cell line | 5–240 µM | Gastric, colon, and lung cancer | [397] |
| Amoora rohituka | Aphanin | C35H54O4 | 538.80 | ↓K-Ras, ↓p-AKT, ↓cMyc, ↓cyclin D1, ↓STAT3, G0/G1 cycle arrest | HPAF-II, BxPC3, and HPAC cells | IC50: 12.80, 15.68 and 17.26 µM | Pancreatic, lung, colon, liver, and skin cancer | [398] |
| Eucalyptus globulus | Caffeic acid | C9H8O4 | 180.16 | ↑ Caspase-1, 3, and 8, G0/G1 cycle arrest | SK-Mel-28 cells | 25–200 µM | Melanoma, breast, colon, gastric, and ovarian cancer | [399] |
| Zingiber officinale | 6-gingerol | C17H26O4 | 294.4 | ↑ROS, ↑Bax/Bcl-2, ↑Caspase-3, 9, ↑cym-c, G2/M phase arrest | AGS cell line | IC50: 250 µM | Liver, lung, breast, and retinoblastoma | [400] |
| Humulus lupulus | Xanthohumol | C21H22O5 | 354.4 | ↑caspase-3, 8 and 9, ↑PARP, ↑p53, ↑AIF, ↓Bcl2, ↓XIAP, S phase cycle arrest | Ca Ski cell line | IC50: 20 µM | Cervical, breast, and mammary adenocarcinoma | [401] |
| Trollius chinensis | Orientin and Vitexin | C21H20O11, C21H20O10 | 448.4, 432.4 | ↑p53, and ↓Bcl2 | EC-109 cells | 5–80 µM | Esophageal, breast, colon, and lung cancer | [402] |
| Rhodiola rosea | Salidroside | C14H20O7 | 300.3 | ↓p-MAPK, ↓p-ERK, ↓p-PI3K, ↓p-AKT, ↓miR99a, ↑p21, ↓Bcl-2 | GES-1, NU-216 and MGC803 cells | 0.8–8000 µM | Gastric and bladder cancer | [403] |
| Syzgium aromaticum | Eugenol | C10H12O2 | 164.2 | ↑ROS, ↑Bax, ↓PCNA, ↓ΔΨm, G2/M cycle arrest | SIHA, SK-MEL-28, A2058, MCF-7, MDA-MB-231 | IC50: <23 µM | Cervical, breast, colon, and lung cancer | [404] |
| Uapaca togoensis | Arborinine | C16H15NO4 | 285.29 | ↓MMP, ↑ROS, G0/G1 and S phase arrest | CCRF-SEM, MDA-MB-231, HCT119, U87 MG, HepG2 cells | IC50: <10 µM | Leukemia, brain, breast, and pancreatic cancer | [405] |
| Peganum harmala | Harmine | C13H12N2O | 212.25 | ↑beclin-1, ↑LC3-II, ↓Bcl-2, ↑Bax, ↓Akt/mTOR, ↓p70S6k | MGC-803 and SGC-7901 cells | IC50: <59 µM | Gastric, breast, ovarian, and pancreatic cancer | [406] |
| Artemisia vestita | Hispidulin | C16H12O6 | 300.26 | ↓VEGFR2, ↓Akt, ↓mTOR, ↓S6 kinase, ↓PI3K, ↓Bcl-2 | PANC-28, BxPC-3, HUVEC, PANC-1 xenograft BALB/c mice | IC50: 20–200 µmol/L | Pancreatic, glioblastoma, and ovarian cancer | [407] |
| Didemnum cuculiferum | Vitilevuamide | C77H114N14O21S | 1603.9 | ↓tubulin polymerization, G2/M phase arrest | A498, HCT116, A5249, and SK-MEL-5 cells and p388 xenograft in mice | LC50: 6–311 nM | Kidney, colon, prostate, ovarian, and pancreatic cancer | [408] |
| Piper longum | Piperlongumine | C17H19NO5 | 317.34 | ↑p-elF2α, ↓ΔΨm, ↑ATF4, ↑CHOP, ↓TrxR1 | SGC-7901, BGC-823, KATO III cell lines | 0.625–20 µM | Gastric, breast, lung, and colon cancer | [409] |
| Chrysanthemum parthenium | Parthenolide | C15H20O3 | 248.32 | ↓NFκβ, ↓VEGF, ↓c-jun, ↓c-Fos | KYSE510, Het-1A, and Eca109 xenograft BALB/c mice | IC50: 13.3, 21.54, and 10.3 µM | Esophageal carcinoma, colon, and prostate cancer | [410] |
| Solanum nigrum L. | Degalactotigonin | C50H82O22 | 1035.2 | ↓Hedgehog Gli1, ↓GSK3β | U2OS, U2OS/MTX, ZOS-M, and ZOS xenograft in mice | IC50: 12.91 –31.46 µmol/L | Osteosarcoma, colon, and pancreatic cancer | [411] |
| Cancer | Bioactives | Class/Family | Combination With Other Drugs | Clinical trial Status | Results | Purpose | References |
|---|---|---|---|---|---|---|---|
| Lung | Curcumin | Phenol | Gefitinib/Erlotinib | Unknown | Not reported | Safety and Tolerability | NCT02321293 * |
| - | - | Lovaza | Ongoing | - | Prevention | NCT03598309 * | |
| Epigallocatechin | Catechin | mLDG | - | - | Treatment | NCT02577393 * | |
| Sulforaphane | Organosulfur | - | - | Prevention | NCT03232138 * | ||
| Gossypol | Phenolic aldehyde | DTX, CIS | Unknown | Not reported | Treatment | NCT01977209* | |
| Breast | ATRA | Retinoid | Anastrozole | Ongoing | - | - | NCT04113863 * |
| Curcumin | Phenol | - | - | - | NCT03980509 * | ||
| - | - | Paclitaxel | Completed | Not posted | - | NCT03072992 * | |
| Genistein | Isoflavone | Gemcitabine | - | Efficacious | Treatment/Prevention | NCT00244933 * | |
| 7-hydroxystaurosporine | Staurosporine derivative | - | Not posted | Treatment | NCT00001444 * | ||
| Emodin | Resin | Unknown | Not posted | Observational | NCT01287468 * | ||
| Cognutrin | Fatty acid + Phenols | Completed | Efficacious | Treatment | NCT01823991 * | ||
| Cervical/Ovarian | Curcumin | Phenol | Cyclophosphamide, Lansoprazole, Aspirin, Vitamin D, Pembrolizumab | Ongoing | - | - | NCT03192059 * |
| OPT-821 | Saponin | Antigen-KLH conjugate Vaccine | Completed | Efficacious | Treatment | NCT00857545 * | |
| Colon | Curcumin | Phenol | 5-FU | Ongoing | - | - | NCT02724202 * |
| - | - | Irinotecan | Completed | Not posted | Toxicity and Pharmacokinetics | NCT01859858 * | |
| - | - | Avastin/FOLFIRI | Completed | Not reported | Treatment | NCT02439385 * | |
| - | - | FOLFOX | Completed | - | - | NCT01490996 * | |
| - | - | Celecoxib | Unknown | - | - | NCT00295035 * | |
| Andrographolide | Diterenoid | Capecitabine | Terminated | Low accrual rate | Treatment | NCT01993472 * | |
| Berberine | Benzylisoquinoline alkaloid | Ongoing | Not posted | Prevention | NCT03281096 * | ||
| Aquamin | Multi-mineral complex | Calcium carbonate | Active | - | - | NCT02647671 * | |
| Cyanidin-3-glucoside | Anthocyanin | Curcumin | Unknown | - | Prevention | NCT01948661 * | |
| Ellagic Acid | Phenol | Completed | - | Treatment | NCT01916239 * | ||
| Silymarin | Flavonolignan | Completed | Unknown | - | NCT03130634 * | ||
| Brain | Chlorogenic acid | Phenol | - | Not posted | Treatment | NCT02728349 * | |
| Perillyl alcohol | Terpenes | Ongoing | - | Treatment | NCT02704858 * | ||
| Head and Neck | Combretastatin A4 Phosphate | Phenol derivative | Completed | - | Treatment | NCT00060242 * | |
| β-Carotene | Terpenoid | α-Tocopherol | - | - | Prevention | NCT00169845 * | |
| Capsaicin | Capsaicinoid | Radiation therapy | - | - | Supportive care | NCT00003610 * | |
| Liver | Silybin | Flavonoid | - | - | Treatment | NCT01129570 * | |
| Prostate | Curcumin | Phenol | - | - | Supportive | NCT01917890 * | |
| - | - | Taxotere | Terminated | No Significant outcome | - | NCT02095717 * | |
| Lycopene | Carotenoid | Vit D3 and E, Selenium, green tea extract | Completed | Not posted | Treatment | NCT00844792 * | |
| Polyphenon E | polyphenol | EGCG | - | Efficacious | Treatment/prevention | NCT00676780 * | |
| Cholecalciferol | Vitamin D | - | Effective | - | NCT00524680 * | ||
| Hematological | Bioperine | Alkaloid | Curcumin | - | - | - | NCT00113841 * |
| Plitidepsin | Depsipeptide | Bortezomib and dexamethasone | - | Not posted | - | NCT02100657 * | |
| Bryostatin 1 | polyketide | - | - | - | NCT00003171 * | ||
| Homoharringtonine | Alkaloid | - | - | - | NCT00006364 * | ||
| Skin cancer | Ingenol Mebutate | Diterpene ester | - | - | - | NCT01325688 * | |
| Advanced solid tumors | Elisidepsin | Depsipeptide | Erlotinib | - | Not posted | - | NCT00884845 * |
| Discodermolide | Polyketide | - | unknown | - | [429] | ||
| Tongue | Luteolin | Flavonoid | Unknown | Not posted | Treatment | NCT03288298 * | |
| GI Tumors | Resveratrol | Phenol | Completed | Not posted | Treatment | NCT01476592 * | |
| Pancreatic | Etoposide | Podophyllotoxin derivative | Gemcitabine | - | - | - | NCT00202800 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. https://doi.org/10.3390/molecules27238367
Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W, Zheng Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules. 2022; 27(23):8367. https://doi.org/10.3390/molecules27238367
Chicago/Turabian StyleNaeem, Abid, Pengyi Hu, Ming Yang, Jing Zhang, Yali Liu, Weifeng Zhu, and Qin Zheng. 2022. "Natural Products as Anticancer Agents: Current Status and Future Perspectives" Molecules 27, no. 23: 8367. https://doi.org/10.3390/molecules27238367
APA StyleNaeem, A., Hu, P., Yang, M., Zhang, J., Liu, Y., Zhu, W., & Zheng, Q. (2022). Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules, 27(23), 8367. https://doi.org/10.3390/molecules27238367