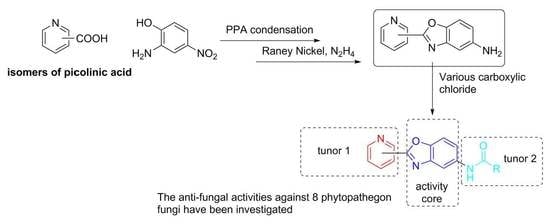
Design, Synthesis, and Anti-Fungal Evaluation of Heterocyclic Benzoxazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. X-ray Crystallographic Studies of 4ac, 4bc
2.3. In Vitro Anti-Fungal Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Synthesis of 3a–3c
3.1.2. General Procedures for the Synthesis of 4aa–4co
N-(2-(Pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4aa)
2-Phenyl-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)acetamide (4ab)
2-Methyl-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4ac)
3-Methyl-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4ad)
4-Methyl-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4ae)
N-(2-(Pyridin-2-yl)benzo[d]oxazol-5-yl)-2-naphthamide (4af)
4-Methoxy-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4ag)
N-(2-(Pyridin-2-yl)benzo[d]oxazol-5-yl)-3-(trifluoromethyl)benzamide (4ah)
N-(2-(Pyridin-2-yl)benzo[d]oxazol-5-yl)-4-(trifluoromethyl)benzamide (4ai)
2-Fluoro-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4aj)
3-Fluoro-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4ak)
4-Fluoro-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4al)
4-Chloro-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4am)
4-Bromo-N-(2-(pyridin-2-yl)benzo[d]oxazol-5-yl)benzamide (4an)
N-(2-(Pyridin-2-yl)benzo[d]oxazol-5-yl)thiophene-2-carboxamide (4ao)
N-(2-(Pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4ba)
2-Phenyl-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)acetamide (4bb)
2-Methyl-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bc)
3-Methyl-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bd)
4-Methyl-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4be)
N-(2-(Pyridin-3-yl)benzo[d]oxazol-5-yl)-2-naphthamide (4bf)
4-Methoxy-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bg)
N-(2-(Pyridin-3-yl)benzo[d]oxazol-5-yl)-3-(trifluoromethyl)benzamide (4bh)
N-(2-(Pyridin-3-yl)benzo[d]oxazol-5-yl)-4-(trifluoromethyl)benzamide (4bi)
2-Fluoro-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bj)
3-Fluoro-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bk)
4-Fluoro-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bl)
4-Chloro-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bm)
4-Bromo-N-(2-(pyridin-3-yl)benzo[d]oxazol-5-yl)benzamide (4bn)
N-(2-(Pyridin-3-yl)benzo[d]oxazol-5-yl)thiophene-2-carboxamide (4bo)
N-(2-(Pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4ca)
2-Phenyl-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)acetamide (4cb)
2-Methyl-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cc)
3-Methyl-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cd)
4-Methyl-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4ce)
N-(2-(Pyridin-4-yl)benzo[d]oxazol-5-yl)-2-naphthamide (4cf)
4-Methoxy-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cg)
N-(2-(Pyridin-4-yl)benzo[d]oxazol-5-yl)-3-(trifluoromethyl)benzamide (4ch)
N-(2-(Pyridin-4-yl)benzo[d]oxazol-5-yl)-4-(trifluoromethyl)benzamide (4ci)
2-Fluoro-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cj)
3-Fluoro-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4ck)
4-Fluoro-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cl)
4-Chloro-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cm)
4-Bromo-N-(2-(pyridin-4-yl)benzo[d]oxazol-5-yl)benzamide (4cn)
N-(2-(Pyridin-4-yl)benzo[d]oxazol-5-yl)thiophene-2-carboxamide (4co)
3.1.3. Structure Determination
3.2. Anti-Fungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ruan, X.; Zhang, C.; Jiang, S.; Guo, T.; Xia, R.; Chen, Y.; Tang, X.; Xue, W. Design, Synthesis, and Biological Activity of Novel Myricetin Derivatives Containing Amide, Thioether, and 1,3,4-Thiadiazole Moieties. Molecules 2018, 23, 3132. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.X.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Li, B.J.; Sun, N.B. Design, Synthesis, DFT Study and Antifungal Activity of Pyrazolecarboxamide Derivatives. Molecules 2016, 21, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, X.K.; Yeong, K.Y. A patent review on the current developments of benzoxazoles in drug discovery. Chem. Med. Chem. 2021, 16, 3237–3262. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kumar, S.; Narasimhan, B.; Lim, S.M.; Ramasamy, K.; Mani, V.; Shah, S.A.A. Design, synthesis and biological potential of heterocyclic benzoxazole scaffolds as promising antimicrobial and anticancer agents. Chem. Cent. J. 2018, 12, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbar, S.S. Most common benzoxazole derivatives as antimicrobial agent (1990–2018). Int. J. Pharm. Technol. 2018, 10, 6695–6712. [Google Scholar]
- Lampa, E.; Romano, A.R.; Berrino, L.; Tortora, G.; Di Guglielmo, R.; Filippelli, A.; Marmo, E. Pharmacological properties of a new non-steroidal anti-inflammatory drug: Flunoxaprofen. Drugs Under Exp. Clin. Res. 1985, 11, 501–509. [Google Scholar]
- Taylor, E.C.; Katz, A.H.; Alvarado, S.I.; McKillop, A. Thallium in Organic Synthesis. 65. A Novel Synthesis of Benzoxazoles from Anilides. J. Org. Chem. 1986, 51, 1607–1609. [Google Scholar] [CrossRef]
- Malamas, M.S.; Manas, E.S.; McDevitt, R.E.; Gunawan, I.; Xu, Z.B.; Collini, M.D.; Miller, C.P.; Dinh, T.; Henderson, R.A.; Keith, J.C.; et al. Design and Synthesis of Aryl Diphenolic Azoles as Potent and Selective Estrogen Receptor-β Ligands. J. Med. Chem. 2004, 47, 5021–5040. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, W.; Han, G.; Qi, J.; Zheng, C.; Zhou, Y.; Ding, L.; Zhao, J.; Zhu, J.; Lv, J.; et al. Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. Eur. J. Med. Chem. 2013, 59, 176–182. [Google Scholar] [CrossRef]
- Das, S.; Mallick, S.; De Sarkar, S. Cobalt-Catalyzed Sustainable Synthesis of Benzimidazoles by Redox-Economical Coupling of o-Nitroanilines and Alcohols. J. Org. Chem. 2019, 84, 12111–12119. [Google Scholar] [CrossRef]
- Zhang, C.; Zhong, B.; Yang, S.; Pan, L.; Yu, S.; Li, Z.; Li, S.; Su, B.; Meng, X. Synthesis and biological evaluation of thiabendazole derivatives as anti-angiogenesis and vascular disrupting agents. Bioorg. Med. Chem. 2015, 23, 3774–3780. [Google Scholar] [CrossRef]
- Zeyrek, C.T.; Arpacı, Ö.T.; Arısoy, M.; Onurdağ, F.K. Synthesis, antimicrobial activity, density functional modelling and molecular docking with COVID-19 main protease studies of benzoxazole derivative: 2-(p-chloro-benzyl)-5-[3-(4-ethly-1-piperazynl) propionamido]-benzoxazole. J. Mol. Struct. 2021, 1237, 130413. [Google Scholar] [CrossRef]
- Temiz-Arpaci, O.; Eylem Cifcioglu Goztepe, B.; Kaynak-Onurdag, F.; Ozgen, S.; Senol, F.; Erdogan Orhan, I. Synthesis and different biological activities of novel benzoxazoles. Acta Biol. Hung. 2013, 64, 249–261. [Google Scholar] [CrossRef]
- Karatas, E.; Foto, E.; Ertan-Bolelli, T.; Yalcin-Ozkat, G.; Yilmaz, S.; Ataei, S.; Zilifdar, F.; Yildiz, I. Discovery of 5-(or 6)-benzoxazoles and oxazolo[4,5-b]pyridines as novel candidate antitumor agents targeting hTopo IIα. Bioorg. Chem. 2021, 112, 104913. [Google Scholar] [CrossRef]
- Huang, S.T.; Hsei, I.J.; Chen, C. Synthesis and anticancer evaluation of bis (benzimidazoles), bis (benzoxazoles), and benzothiazoles. Biorg. Med. Chem. 2006, 14, 6106–6119. [Google Scholar] [CrossRef]
- Kumar, D.; Jacob, M.R.; Reynolds, M.B.; Kerwin, S.M. Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1. Biorg. Med. Chem. 2002, 10, 3997–4004. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Singh, N.; Kumar, A.; Lozach, O.; Meijer, L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Biorg. Med. Chem. 2006, 14, 3758–3765. [Google Scholar] [CrossRef]
- Paderni, D.; Giorgi, L.; Voccia, M.; Formica, M.; Caporaso, L.; Macedi, E.; Fusi, V. A new benzoxazole-based fluorescent macrocyclic chemosensor for optical detection of Zn2+ and Cd2+. Chemosensors 2022, 10, 188. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, K.; Chen, P.; Gao, A.; Du, W.; Chen, X.; An, Z. Synthesis and properties of mesogenic laterally fluorinated compounds containing benzoxazole unit. Liq. Cryst. 2014, 41, 1042–1056. [Google Scholar] [CrossRef]
- Bansal, S. Recent advances in synthesis of benzoxazole. Mini-Rev. Org. Chem. 2021, 18, 383–397. [Google Scholar] [CrossRef]
- Xing, R.G.; Li, Y.N. Progress in synthesis of 2-substituted benzoxazoles. Chin. J. Org. Chem. 2014, 36, 131–135. [Google Scholar]
- Arisoy, M.; Temiz-Arpaci, O.; Yildiz, I.; Kaynak-Onurdag, F.; Aki, E.; Yalcin, I.; Abbasoglu, U. Synthesis, antimicrobial activity and QSAR studies of 2,5-disubstituted benzoxazoles. SAR QSAR Environ. Res. 2008, 19, 589–612. [Google Scholar] [CrossRef] [PubMed]
- Rida, S.M.; Ashour, F.A.; El-Hawash, S.A.M.; ElSemary, M.M.; Badr, M.H.; Shalaby, M.A. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Si, C.; Li, Y.; Wang, Y.; Lu, J. Improved synthesis of 5-substituted 1H-tetrazoles via the 3+2 cycloaddition of nitriles and sodium azide catalyzed by silica sulfuric acid. Int. J. Mol. Sci. 2012, 13, 4696–4703. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Huang, L.Z.; Wu, J.; Lu, D.; Ma, B.L.; Du, Z.T. Microwave-assisted synthesis of 2-substituted 1h-benzo[d]imidazoles and their antifungal activities in vitro. Heterocycles 2013, 87, 1545–1550. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, L.Z.; Li, Y.-Q.; Du, Z.T. Synthesis of substituted 6H-benzo[c]chromenes: A palladium promoted ring closure of diazonium tetrafluoroborates. Tetrahedron Lett. 2012, 53, 7036–7070. [Google Scholar] [CrossRef]
- Li, C.; Zhong, J.; Liu, B.; Yang, T.; Lv, B.; Luo, Y. Study on Typical Diarylurea Drugs or Derivatives in Cocrystallizing with Strong H-Bond Acceptor DMSO. ACS Omega 2021, 6, 5532–5547. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, D.; Li, B.; Zhang, B.; Miao, F.; Zhou, L. Bioactivity and structure-activity relationship of cinnamic acid esters and their derivatives as potential antifungal agents for plant protection. PLoS ONE 2017, 12, e0176189. [Google Scholar] [CrossRef]






 | ||||
|---|---|---|---|---|
| Entry | 2-Pyridyl | 3-Pyridyl | 4-Pyridyl | Ra |
| 1 | 4aa | 4ba | 4ca |  |
| 2 | 4ab | 4bb | 4cb |  |
| 3 | 4ac | 4bc | 4cc |  |
| 4 | 4ad | 4bd | 4cd |  |
| 5 | 4ae | 4be | 4ce |  |
| 6 | 4af | 4bf | 4cf |  |
| 7 | 4ag | 4bg | 4cg |  |
| 8 | 4ah | 4bh | 4ch |  |
| 9 | 4ai | 4bi | 4ci |  |
| 10 | 4aj | 4bj | 4cj |  |
| 11 | 4ak | 4bk | 4ck |  |
| 12 | 4al | 4bl | 4cl |  |
| 13 | 4am | 4bm | 4cm |  |
| 14 | 4an | 4bn | 4cn |  |
| 15 | 4ao | 4bo | 4co |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Kang, R.; Yang, X.; Cheng, Y.; Bai, H.; Du, Z. Design, Synthesis, and Anti-Fungal Evaluation of Heterocyclic Benzoxazole Derivatives. Molecules 2022, 27, 8375. https://doi.org/10.3390/molecules27238375
Wang R, Kang R, Yang X, Cheng Y, Bai H, Du Z. Design, Synthesis, and Anti-Fungal Evaluation of Heterocyclic Benzoxazole Derivatives. Molecules. 2022; 27(23):8375. https://doi.org/10.3390/molecules27238375
Chicago/Turabian StyleWang, Ruibo, Ruiting Kang, Xuan Yang, Yu Cheng, Hongjin Bai, and Zhenting Du. 2022. "Design, Synthesis, and Anti-Fungal Evaluation of Heterocyclic Benzoxazole Derivatives" Molecules 27, no. 23: 8375. https://doi.org/10.3390/molecules27238375
APA StyleWang, R., Kang, R., Yang, X., Cheng, Y., Bai, H., & Du, Z. (2022). Design, Synthesis, and Anti-Fungal Evaluation of Heterocyclic Benzoxazole Derivatives. Molecules, 27(23), 8375. https://doi.org/10.3390/molecules27238375