Partial Characterization of Lectins Purified from the Surco and Vara (Furrow and Rod) Varieties of Black Phaseolus vulgaris
Abstract
:1. Introduction
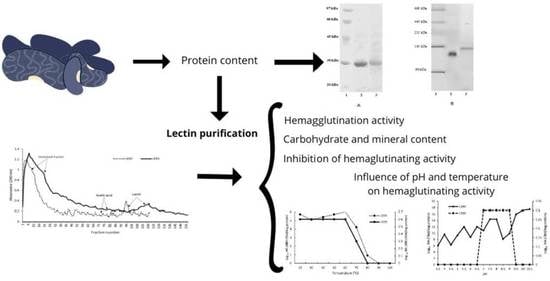
2. Results
2.1. Protein Content
2.2. Lectin Purification
2.3. Polyacrylamide Gel Eletrophoresis (SDS-PAGE and NATIVE-PAGE)
2.4. Hemagglutination Activity
2.5. Carbohydrate and Mineral Content
2.6. Inhibition of Hemaglutinating Activity
2.7. Influence of pH and Temperature on Hemaglutinating Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Protein Quantification
4.4. Lectin Extraction
4.5. Lectin Purification
4.6. Hemagglutinating Activity
4.7. Hemagglutination Inhibition
4.8. Polyacrylamide Gel Electrophoresis (PAGE)
4.9. Carbohydrate and Metal Ion Content Determination
4.10. Effect of Metal Ions on Hemagglutination
4.11. Influence of pH and Temperature on Hemagglutination
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant Activity in Common Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Maldonado, H.; Paredes-López, O. Production of High-Protein Flour and Maltodextrins from Amaranth Grain. Process Biochem. 1994, 29, 289–293. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Tyler, R.T.; Toews, R.; Gawalko, E.J. Effect of Cooking on the Composition of Beans (Phaseolus vulgaris L.) and Chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. History of Lectins: From Hemagglutinins to Biological Recognition Molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [Green Version]
- Soumya Purkait, S.K. International Journal of Health Sciences and Research. Int. J. Health Sci. Res. 2019, 9, 156–164. [Google Scholar]
- Valadez-Vega, C.; Guzmán-Partida, A.M.; Soto-Cordova, F.J.; Álvarez-Manilla, G.; Morales-González, J.A.; Madrigal-Santillán, E.; Villagómez-Ibarra, J.R.; Zúniga-Pérez, C.; Gutiérrez-Salinas, J.; Becerril-Flores, M.A. Purification, Biochemical Characterization, and Bioactive Properties of a Lectin Purified from the Seeds of White Tepary Bean (Phaseolus Acutifolius Variety Latifolius). Molecules 2011, 16, 2561–2582. [Google Scholar] [CrossRef] [Green Version]
- Qadir, S.; Hussain Wani, I.; Rafiq, S.; Ahmad Ganie, S.; Masood, A.; Hamid, R. Evaluation of Antimicrobial Activity of a Lectin Isolated and Purified from <I>Indigofera Heterantha. Adv. Biosci. Biotechnol. 2013, 4, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Swarna, R.R.; Asaduzzaman, A.K.M.; Kabir, S.R.; Arfin, N.; Kawsar, S.M.A.; Rajia, S.; Fujii, Y.; Ogawa, Y.; Hirashima, K.; Kobayashi, N.; et al. Antiproliferative and Antimicrobial Potentials of a Lectin from Aplysia Kurodai (Sea Hare) Eggs. Mar. Drugs 2021, 19, 394. [Google Scholar] [CrossRef]
- González-Cruz, L.; Valadez-Vega, C.; Juárez-Goiz, J.M.S.; Flores-Martínez, N.L.; Montañez-Soto, J.L.; Bernardino-Nicanor, A. Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus Coccineus (Ayocote Bean). Agronomy 2022, 12, 716. [Google Scholar] [CrossRef]
- Stauder, H.; Kreuser, E.D. Mistletoe Extracts Standardised in Terms of Mistletoe Lectins (ML I) in Oncology: Current State of Clinical Research. Onkologie 2002, 25, 374–380. [Google Scholar] [CrossRef]
- Ibernon, M.; Moreso, F.; Serón, D. Innate Immunity in Renal Transplantation: The Role of Mannose-Binding Lectin. Transplant. Rev. 2014, 28, 21–25. [Google Scholar] [CrossRef]
- Sharon, N. Lectins: Past, Present and Future. Biochem. Soc. Trans. 2008, 36, 1457–1460. [Google Scholar] [CrossRef]
- Fang, E.F.; Lin, P.; Wong, J.H.; Tsao, S.W.; Ng, T.B. A Lectin with Anti-HIV-1 Reverse Transcriptase, Antitumor, and Nitric Oxide Inducing Activities from Seeds of Phaseolus vulgaris Cv. Extralong Autumn Purple Bean. J. Agric. Food Chem. 2010, 58, 2221–2229. [Google Scholar] [CrossRef]
- Van Damme, E.J.M.; Rouge, P.; Peumans, W.J. 3.26 Plant Lectins. Elsevier: Gent, België, 2007; pp. 563–599. [Google Scholar]
- Armienta-A, E.; Moreno-Leg, M.; Armienta-A, E.; Laguna-Gra, S.V. Partial Characterization of the Lectin of Runner Beans (Phaseolus Coccieneus) Var. Alubia. Pakistan J. Biol. Sci. 2009, 12, 459–462. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Chen, T.T.; Hu, L.; Yang, J.; Hu, P.; Wang, S.Y. Purification and Characterization of a Novel Lectin from Chinese Leek Seeds. J. Agric. Food Chem. 2015, 63, 1488–1495. [Google Scholar] [CrossRef]
- Sharma, A.; Ng, T.B.; Wong, J.H.; Lin, P. Purification and Characterization of a Lectin from Phaseolus vulgaris Cv. (Anasazi Beans). J. Biomed. Biotechnol. 2009, 2009, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Loris, R.; Hamelryck, T.; Bouckaert, J.; Wyns, L. Legume Lectin Structure. Biochim. Et Biophys. Acta-Protein Struct. Mol. Enzymol. 1998, 1383, 9–36. [Google Scholar] [CrossRef]
- Franz, H.; Ziska, P.; Mohr, J. Lectins—Definition and Classification. Acta Histochem. 1982, 71, 19–21. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Wang, S.; Rao, P. Lunatin, a Novel Lectin with Antifungal and Antiproliferative Bioactivities from Phaseolus Lunatus Billb. Int. J. Biol. Macromol. 2016, 89, 717–724. [Google Scholar] [CrossRef]
- El-Araby, M.M.; El-Shatoury, E.H.; Soliman, M.M.; Shaaban, H.F. Characterization and Antimicrobial Activity of Lectins Purified from Three Egyptian Leguminous Seeds. AMB Express 2020, 10, 90. [Google Scholar] [CrossRef]
- Sparvoli, F.; Lanave, C.; Santucci, A.; Bollini, R.; Lioi, L. Lectin and Lectin-Related Proteins in Lima Bean (Phaseolus lunatus L.) Seeds: Biochemical and Evolutionary Studies. Plant Mol. Biol. 2001, 45, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; González De Mejía, E.; Loarca-Piña, G. Purification and Acute Toxicity of a Lectin Extracted from Tepary Bean (Phaseolus Acutifolius). Food Chem. Toxicol. 2003, 41, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Villanueva, A.; Caballero-Ortega, H.; Abdullaev-Jafarova, F.; Garfias, Y.; del Carmen Jiménez-Martínez, M.; Bouquelet, S.; Martínez, G.; Mendoza-Hernández, G.; Zenteno, E. Lectin from Phaseolus Acutifolius Var. Escumite: Chemical Characterization, Sugar Specificity, and Effect on Human T-Lymphocytes. J. Agric. Food Chem. 2007, 55, 5781–5787. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, B.; Ji, N.; Zhou, J.; Bian, H.J.; Li, C.Y.; Chen, F.; Bao, J.K. A Novel Sialic Acid-Specific Lectin from Phaseolus Coccineus Seeds with Potent Antineoplastic and Antifungal Activities. Phytomedicine 2009, 16, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wong, J.; Lin, P.; Chan, Y.; Ng, T. Purification and Characterization of a Lectin from the Indian Cultivar of French Bean Seeds. Protein Pept. Lett. 2010, 17, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Nciri, N.; Cho, N.; El Mhamdi, F.; Ismail, H.B.; Mansour, A.B.; Sassi, F.H.; Aissa-Fennira, F. Ben. Toxicity Assessment of Common Beans (Phaseolus vulgaris L.) Widely Consumed by Tunisian Population. J. Med. Food 2015, 18, 1049–1064. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Walid, E.; Ma, Y.; Xue, S.J. Extraction and Purification of a Lectin from Small Black Kidney Bean (Phaseolus vulgaris) Using a Reversed Micellar System. Process Biochem. 2013, 48, 746–752. [Google Scholar] [CrossRef]
- Adenike, K.; Eretan, O.B. Purification and Partial Characterization of a Lectin from the Fresh Leaves of Kalanchoe Crenata (Andr.) Haw. J. Biochem. Mol. Biol. 2004, 37, 229–233. [Google Scholar] [CrossRef]
- Munonye, N.O.J. Comparative Evaluation of the Proximate Composition, Anti-Nutrients and Functional Properties of Some Underutilized Pulses. Food Sci. Qual. Manag. 2020, 95, 72–76. [Google Scholar] [CrossRef]
- Heinemann, A.B.; Ramirez-Villegas, J.; Souza, T.L.P.O.; Didonet, A.D.; di Stefano, J.G.; Boote, K.J.; Jarvis, A. Drought Impact on Rainfed Common Bean Production Areas in Brazil. Agric. For. Meteorol. 2016, 225, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.S.; Barros, A.I.R.N.A. Nutrients, Antinutrients, Phenolic Composition, and Antioxidant Activity of Common Bean Cultivars and Their Potential for Food Applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.; Bacilio, M.; Porras, F.; Juarez, S.; Debray, H.; Zenteno, E.; Ortiz, B. A Comparative Study on the Purification of the Amaranthus Leucocarpus Syn. Hypocondriacus Lectin. Prep. Biochem. Biotechnol. 1999, 29, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Zenteno, E.; Ochoa, J.L. Purification of a Lectin from Amaranthus Leucocarpus by Affinity Chromatography. Phytochemistry 1988, 27, 313–317. [Google Scholar] [CrossRef]
- Valadez-Vega, C.; Lugo-Magaña, O.; Morales-González, J.A.; Delgado-Olivares, L.; Izquierdo-Vega, J.A.; Sánchez-Gutiérrez, M.; López-Contreras, L.; Bautista, M.; Velázquez-González, C. Phytochemical, Cytotoxic, and Genotoxic Evaluation of Protein Extract of Amaranthus Hypochondriacus Seeds. CYTA—J. Food 2021, 19, 701–709. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Ilic, S.; Jun Xue, S.; Kakuda, Y. Biological Properties and Characterization of Lectin from Red Kidney Bean (Phaseolus vulgaris). Food Rev. Int. 2009, 25, 12–27. [Google Scholar] [CrossRef]
- Singh, H.; Sarathi, S.P. Insight of Lectins—A Review. Int. J. Sci. Eng. Res. 2012, 3, 1–9. Available online: https://www.ijser.org/paper/Insight-of-Lectins-A-review.html (accessed on 15 July 2020).
- Sathe, S.K. Dry Bean Protein Functionality. Crit. Rev. Biotechnol. 2002, 22, 175–223. [Google Scholar] [CrossRef]
- Nciri, N.; Cho, N.; El Mhamdi, F.; Ben Mansour, A.; Haj Sassi, F.; Ben Aissa-Fennira, F. Identification and Characterization of Phytohemagglutinins from White Kidney Beans (Phaseolus vulgaris L., Var. Beldia) in the Rat Small Intestine. J. Med. Food 2016, 19, 85–97. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; de la Fuente, G.; Agundis, C.; Córdoba, F.; Vargas-Albores, F.; de la Fuente, G.; Agundis, C.; Córdoba, F. Purification and Characterization of a Lectin from Phaseolus Acutifolius Var. Latifolius. Prep. Biochem. 1987, 17, 379–396. [Google Scholar] [CrossRef]
- Khan, F.; Khan, R.H.; Sherwani, A.; Mohmood, S.; Azfer, M.A. Lectins as Markers for Blood Grouping. Med. Sci. Monit. 2002, 8, RA293-300. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12503049 (accessed on 15 July 2020).
- Tsaneva, M.; Van Damme, E.J.M. 130 Years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Bernardes, E.S.; Rosa, C.; Faca, V.; Greene, L.J.; Ward, R.J. Isolation, Purification, and Physicochemical Characterization of a D-Galactose-Binding Lectin from Seeds of Erythrina Speciosa. Arch. Biochem. Biophys. 2003, 410, 222–229. [Google Scholar] [CrossRef]
- Ellen, R.P.; Fillery, E.D.; Chan, K.H.; Grove, D.A. Sialidase-Enhanced Lectin-like Mechanism for Actinomyces Viscosus and Actinomyces Naeslundii Hemagglutination. Infect. Immun. 1980, 27, 335–343. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Hasan, I.; Chakrabortty, A.; Zaman, S.; Islam, S.S.; Ahmed, F.R.S.; Kabir, K.M.A.; Nurujjaman, M.; Uddin, M.B.; Alam, M.T.; et al. Moringa Oleifera Seed Lectin Inhibits Ehrlich Ascites Carcinoma Cell Growth by Inducing Apoptosis through the Regulation of Bak and NF-ΚB Gene Expression. Int. J. Biol. Macromol. 2018, 107, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Pan, W.L.; Wong, J.H.; Chan, Y.S.; Ye, X.J.; Ng, T.B. A New Phaseolus vulgaris Lectin Induces Selective Toxicity on Human Liver Carcinoma Hep G2 Cells. Arch. Toxicol. 2011, 85, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Partida, A.M.; Robles-Burgueño, M.R.; Ortega-Nieblas, M.; Vázquez-Moreno, I. Purification and Characterization of Complex Carbohydrate Specific Isolectins from Wild Legume Seeds: Acacia Constricta Is (Vinorama) Highly Homologous to Phaseolus vulgaris Lectins. Biochimie 2004, 86, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, W.G.; Levy, A.; González, D.I. Isolation and Partial Characterization of Bean Phytohemagglutinins. Phytochemistry 1974, 13, 2685–2693. [Google Scholar] [CrossRef]
- Ohtani, K.; Shibata, S.; Misaki, A. Purification and Characterization of Tora-Bean (Phaseolus vulgaris) Lectin1. J. Biochem. 1980, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cavada, B.S.; Bari, A.U.; Pinto-Junior, V.R.; Lossio, C.F.; Silva, M.T.L.; Souza, L.A.G.; Oliveira, M.V.; Souza-Filho, C.H.D.; Correia, S.E.G.; Vital, A.P.M.S.; et al. Purification and Partial Characterization of a New Lectin from Parkia Panurensis Benth. Ex H.C. Hopkins Seeds (Leguminosae Family; Mimosoideae Subfamily) and Evaluation of Its Biological Effects. Int. J. Biol. Macromol. 2020, 145, 845–855. [Google Scholar] [CrossRef]
- Bonnardel, F.; Mariethoz, J.; Salentin, S.; Robin, X.; Schroeder, M.; Perez, S.; Lisacek, F.D.S.; Imberty, A. Unilectin3d, a Database of Carbohydrate Binding Proteins with Curated Information on 3D Structures and Interacting Ligands. Nucleic Acids Res. 2019, 47, D1236–D1244. [Google Scholar] [CrossRef] [Green Version]
- Leal, R.B.; Pinto-Junior, V.R.; Osterne, V.J.S.; Wolin, I.A.V.; Nascimento, A.P.M.; Neco, A.H.B.; Araripe, D.A.; Welter, P.G.; Neto, C.C.; Correia, J.L.A.; et al. Crystal Structure of DlyL, a Mannose-Specific Lectin from Dioclea Lasiophylla Mart. Ex Benth Seeds That Display Cytotoxic Effects against C6 Glioma Cells. Int. J. Biol. Macromol. 2018, 114, 64–76. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Lv, X.; Li, D.; Liu, C.; Feng, Z. Two-Step Isolation, Purification, and Characterization of Lectin from Zihua Snap Bean (Phaseolus vulgaris) Seeds. Polymers 2019, 11, 785. [Google Scholar] [CrossRef] [Green Version]
- Gautam, A.K.; Sharma, D.; Sharma, J.; Saini, K.C. Legume Lectins: Potential Use as a Diagnostics and Therapeutics against the Cancer. Int. J. Biol. Macromol. 2020, 142, 474–483. [Google Scholar] [CrossRef]
- Pinto, V.R.; De Santiago, M.Q.; Da Silva Osterne, V.J.; Correia, J.L.A.; Pereira, F.N.; Cajazeiras, J.B.; De Vasconcelos, M.A.; Teixeira, E.H.; Do Nascimento, A.S.F.; Miguel, T.B.A.R.; et al. Purification, Partial Characterization and Immobilization of a Mannose-Specific Lectin from Seeds of Dioclea Lasiophylla Mart. Molecules 2013, 18, 10857–10869. [Google Scholar] [CrossRef] [Green Version]
- Abhilash, J.; Dileep, K.V.; Palanimuthu, M.; Geethanandan, K.; Sadasivan, C.; Haridas, M. Metal Ions in Sugar Binding, Sugar Specificity and Structural Stability of Spatholobus Parviflorus Seed Lectin. J. Mol. Model. 2013, 19, 3271–3278. [Google Scholar] [CrossRef]
- Fred Brewer, C.; Brown, R.D.; Koenig, S.H. Metal Ion Binding and Conformational Transitions in Concanavalin a: A Structure-Function Study. J. Biomol. Struct. Dyn. 1983, 1, 961–997. [Google Scholar] [CrossRef]
- Zhao, J.K.; Wang, H.X.; Ng, T.B. Purification and Characterization of a Novel Lectin from the Toxic Wild Mushroom Inocybe Umbrinella. Toxicon 2009, 53, 360–366. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Shao, J.; Jia, J.; Tian, Y.; Shu, X.; Ren, X.; Guan, Y. Extraction, Purification and Physicochemical Properties of a Novel Lectin from Laetiporus Sulphureus Mushroom. Lwt 2018, 91, 151–159. [Google Scholar] [CrossRef]
- Rodrigues, R.; Silva, E.; Thalles, J.; Gomes, J.; Lacerda, D.; Pinto, S.; Rizzi, C.; Marques, M.; Ribeiro, I.; Mateus, S.; et al. Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int. J. Biol. Macromol. 2016, 95, 1072–1081. [Google Scholar] [CrossRef]
- Lavanya Latha, V.; Nagender Rao, R.; Nadimpalli, S.K. Affinity Purification, Physicochemical and Immunological Characterization of a Galactose-Specific Lectin from the Seeds of Dolichos Lablab (Indian Lablab Beans). Protein Expr. Purif. 2006, 45, 296–306. [Google Scholar] [CrossRef]
- Singh, R.S.; Thakur, S.R.; Kennedy, J.F. Purification and Characterisation of a Xylose-Specific Mitogenic Lectin from Fusarium Sambucinum. Int. J. Biol. Macromol. 2020, 152, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K.; Kennedy, J.F. Purification and Characterization of a Mitogenic Lectin from Penicillium Duclauxii. Int. J. Biol. Macromol. 2018, 116, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.; Siddiq, M.; Harte, J.B.; Dolan, K.D.; Nyombaire, G.; Suniaga, H. Use of Low-Temperature Extrusion for Reducing Phytohemagglutinin Activity (PHA) and Oligosaccharides in Beans (Phaseolus vulgaris L.) Cv. Navy and Pinto. Food Chem. 2012, 133, 1636–1639. [Google Scholar] [CrossRef]
- Jebor, M.A.; Yasir, A. Extraction, Purification and Characterization of a Lectin from White Kidney Bean. Med. J. Babylon 2012, 9, 11. Available online: https://www.iasj.net/iasj/download/c703b219cc02ce70 (accessed on 15 July 2022).
- Koch, W.; Czop, M.; Iłowiecka, K.; Nawrocka, A.; Wiącek, D. Dietary Intake of Toxic Heavy Metals with Major Groups of Food Products-Results of Analytical Determinations. Nutrients 2022, 14, 1626. [Google Scholar] [CrossRef]
- Shi, J.; Xue, S.J.; Kakuda, Y.; Ilic, S.; Kim, D. Isolation and Characterization of Lectins from Kidney Beans (Phaseolus vulgaris). Process Biochem. 2007, 42, 1436–1442. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Huang, Y.; Li, M.; Lu, J.; Jin, N.; He, Y.; Fan, B. Phytohemagglutinin Content in Fresh Kidney Bean in China. Int. J. Food Prop. 2019, 22, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Ricardo, J. Solá and Kai Griebenow. Effects of Glycosylation on the Stability of Protein Pharmaceuticals. Mol. Cell. Biochem. 2009, 23, 1–7. [Google Scholar] [CrossRef]
- Cunniff, P.A. Official Methods of Analysis of AOAC International. Assoc. Off. Anal. Chem. Int. 1976, 9, 471. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [CrossRef]
- Jaffé, W.G.; Brucher, O. Detection of four types of specific phytohemagglutinins in different lines beans (Phaseolus vulgaris). Z. Immun.-Forsch. Bd 1972, 447, 439–447. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]



| Fraction | Protein Concentration (mg/mL) | Hemagglutination Titer * (Units) | Specific Activity (HA) | Purification Factor | Lectin (%) |
|---|---|---|---|---|---|
| BBV | |||||
| Crude extract | 1086.47 | 16 | 0.0147 | 1 | |
| Protein bound to stroma | 0.0488 | 8 | 163.93 | 11,151.7 | 0.0045 a |
| BBS | |||||
| Crude extract | 1034.77 | 1.09 × 1012 | 1.05 × 109 | 1 | |
| Protein bound to stroma | 0.1062 | 8.38 × 106 | 7.89 × 107 | 0.075 | 0.0103 b |
| Erythrocyte Type | Lectins | |
|---|---|---|
| Human | BBV | BBS |
| Hemagglutination Titer | ||
| A | 16 ± 0 a | 2097152 ± 0 a |
| B | 16 ± 0 a | 67108864 ± 0 b |
| O | 4 ± 0 b | 4.39 × 1012 ± 0 c |
| Animals | ||
| Rabbit | 128 ± 0 a | 134217728 ± 0 a |
| Chicken | 32 ± 0 b | 262144 ± 0 b |
| Hamster | 1.12 × 1015 ± 0 c | 7.92 × 1028 ± 0 c |
| Bull | ND | 1024 ± 0 d |
| Pig | 16 ± 0 d | 524288 ± 0 e |
| Sheep | 2 ± 0 e | 8192 ± 0 f |
| Metal | Native Lectins | Demetallized Lectins | ||
|---|---|---|---|---|
| BBV | BBS | BBV | BBS | |
| Concentration (ppm) | ||||
| Ca+2 | 597.5 ± 0.03 a | 823.0 ± 0.05 a | 284.0 ± 0.01 b | 199.0 ± 0.02 b |
| Cu−1 | 274.5 ± 0.02 a | 212.5 ± 0.01 a | 185.0 ± 0.01 b | 186.0 ± 0.02 b |
| Mg+2 | 487.0 ± 0.03 a | 774.5 ± 0.25 a | 358.0 ± 0.02 b | 171.0 ± 0.06 b |
| Mn+2 | 224.0 ± 0.01 a | 176.5 ± 0.02 a | 185.0 ± 0.01 b | 170.0 ± 0.02 a |
| Zn+2 | 306.5 ± 0.02 a | 316.5 ± 0.08 a | 182.5 ± 0.01 b | 182.0 ± 0.02 b |
| Native Lectin | Demetallized Lectin | Demetallized Lectins Reconstituted with Metals (Hemagglutination Titer) | |||
|---|---|---|---|---|---|
| Hemagglutination Titer | Ca2+ | Mg2+ | Mn2+ | ||
| BBV | 2 ± 0 a | 0 ± 0 a | 128 ± 0 a | 4096 ± 0 a | 4 ± 0 a |
| BBS | 1,073,741,824 ± 0 b | 16 ± 0 b | 6.87 × 1010 ± 0 b | 1.09 × 1012 ± 0 b | 2048 ± 0 b |
| Inhibitory Concentration (mg/mL) * | ||
|---|---|---|
| Monosaccharides | LBBV | LBBS |
| Glucose | ND | ND |
| Galactose | ND | ND |
| Mannose | ND | 45.04 |
| Fructose | ND | ND |
| Oligosaccharides | ||
| Maltose | ND | ND |
| Trehalose | ND | ND |
| Raffinose | 252.21 | ND |
| Glycoproteins | ||
| Ovoalbumin | ND | ND |
| Fetuin | 0.0075 | 0.129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valadez-Vega, C.; Lugo-Magaña, O.; Betanzos-Cabrera, G.; Villagómez-Ibarra, J.R. Partial Characterization of Lectins Purified from the Surco and Vara (Furrow and Rod) Varieties of Black Phaseolus vulgaris. Molecules 2022, 27, 8436. https://doi.org/10.3390/molecules27238436
Valadez-Vega C, Lugo-Magaña O, Betanzos-Cabrera G, Villagómez-Ibarra JR. Partial Characterization of Lectins Purified from the Surco and Vara (Furrow and Rod) Varieties of Black Phaseolus vulgaris. Molecules. 2022; 27(23):8436. https://doi.org/10.3390/molecules27238436
Chicago/Turabian StyleValadez-Vega, Carmen, Olivia Lugo-Magaña, Gabriel Betanzos-Cabrera, and José Roberto Villagómez-Ibarra. 2022. "Partial Characterization of Lectins Purified from the Surco and Vara (Furrow and Rod) Varieties of Black Phaseolus vulgaris" Molecules 27, no. 23: 8436. https://doi.org/10.3390/molecules27238436
APA StyleValadez-Vega, C., Lugo-Magaña, O., Betanzos-Cabrera, G., & Villagómez-Ibarra, J. R. (2022). Partial Characterization of Lectins Purified from the Surco and Vara (Furrow and Rod) Varieties of Black Phaseolus vulgaris. Molecules, 27(23), 8436. https://doi.org/10.3390/molecules27238436