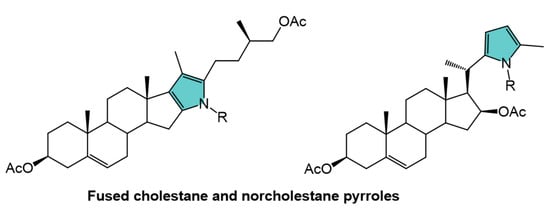
Fused Pyrroles in Cholestane and Norcholestane Side Chains: Acaricidal and Plant Growth-Promoting Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
2.2. NMR Characterization
2.3. Biological Evaluation
2.3.1. Acaricidal Activity against the Two-Spotted Spider Mite (Tetranychus urticae Koch)
2.3.2. Plant Growth Evaluation on Habanero Pepper (Capsicum chinense Jacq)
3. Materials and Methods
3.1. General Remarks
3.2. General Procedures for the Synthesis of Pyrroles
3.2.1. Conventional Heating Methodology
3.2.2. Microwave Methodology
3.3. (25R)-N-benzylpyrrolo[2’,3’,4’,5’:16,17,20,22]cholest-5-ene-3β,26-diyl diacetate (3a)
3.4. (25R)-N-(2-hydroxy)ethylpyrrolo[2’,3’,4’,5’:16,17,20,22]cholest-5-ene-3β,26-diyl diacetate (3b)
3.5. (25R)-N-(3-hydroxy)propylpyrrolo[2’,3’,4’,5’:16,17,20,22]cholest-5-ene-3β,26-diyl diacetate (3c)
3.6. (25R)-22,25-dioxo-27-norcholest-5-en-3β,16-diyl diacetate (7)
3.7. N-Benzylpyrrolo [2′,3′,4′,5′:22,23,24,25]-27-Norcholest-5-ene 3β,16-Diyl Diacetate (8a)
3.8. N-(2-hydroxy)ethylpyrrolo[2’,3’,4’,5’:22,23,24,25]-27-norcholest-5-ene 3β,16-diyl diacetate (8b)
3.9. N-(3-hydroxy)propylpyrrolo[2’,3’,4’,5’:22,23,24,25]-27-norcholest-5-ene 3β,16-diyl diacetate (8c)
3.10. General Remarks for Bioassays
3.11. Acaricidal Activity in the Two-Spotted Spider Mite (Tetranychus urticae Koch) under Laboratory Conditions
3.11.1. Bioassay for T. urticae Adults
3.11.2. Bioassay for T. urticae Eggs
3.12. Plant Growth Promotion in Habanero Pepper Plants (Capsicum chinense Jacq) under Greenhouse Conditions
3.12.1. Establishment of Potted Plants
3.12.2. Compound Application and Evaluation
3.13. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Gossauer, A. Pyrrole als Naturprodukte. In Die Chemie der Pyrrole; Gossauer, A., Ed.; Organische Chemie in Einzeldarstellungen; Springer: Berlin/Heidelberg, Germany, 1974; pp. 189–208. ISBN 978-3-642-51118-9. [Google Scholar]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A Resourceful Small Molecule in Key Medicinal Hetero-Aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Jones, R.A.; Bean, G.P. The Chemistry of Pyrroles: Organic Chemistry: A Series of Monographs; Academic Press: Cambridge, MA, USA, 2013; Volume 34, ISBN 978-1-4832-1847-2. [Google Scholar]
- Trofimov, B.A.; Al’bina, I.M.; Schmidt, E.Y.; Sobenina, L.N. Chemistry of Pyrroles; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1-4822-3242-1. [Google Scholar]
- Fleischer, E.B. Structure of Porphyrins and Metalloporphyrins. Acc. Chem. Res. 1970, 3, 105–112. [Google Scholar] [CrossRef]
- Kephart, J.C. Chlorophyll Derivatives—Their Chemistry? Commercial Preparation and Uses. Econ. Bot. 1955, 9, 3–38. [Google Scholar] [CrossRef]
- Falk, H. The Chemistry of Linear Oligopyrroles and Bile Pigments; Monatshefte für Chemie/Chemical Monthly Supplementa; Springer: Vienna, Austria, 1989; Volume 1, ISBN 978-3-7091-7441-8. [Google Scholar]
- Khaghaninejad, S.; Heravi, M.M. Paal–Knorr Reaction in the Synthesis of Heterocyclic Compounds. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 111, pp. 95–146. [Google Scholar]
- Vladimirova, S.P.; Danalev, D.L.; Marinkova, D.A.; Raykova, R.N.; Manova, D.S.; Marinova, S.R.; Marinova, D.I.; Yaneva, S.A. Synthesis and Antibacterial Activity of Amino Acids Modified with Specifically Substituted Pyrrole Heterocycle. Bulg. Chem. Commun. 2017, 49, 86–89. [Google Scholar]
- Danalev, D.L.; Vladimirova, S.P.; Borisov, B.P.; Nocheva, H.H.; Bocheva, A.I.; Marinkova, D.A.; Naydenova, E.D.; Lozanov, V.S. Synthesis and Analgesic Activity of New Analogues of Tyr-MIF Including Pyrrole Moiety. Int. J. Pept. Res. Ther. 2016, 22, 243–248. [Google Scholar] [CrossRef]
- Barton, D.; Kervagoret, J.; Zard, S.Z. A Useful Synthesis of Pyrroles from Nitroolefins. Tetrahedron 1990, 46, 7587–7598. [Google Scholar] [CrossRef]
- Zaitsev, A.B.; Vasil’tsov, A.M.; Shmidt, E.Y.; Mikhaleva, A.I.; Afonin, A.V.; Il’icheva, L.N. Trofimov Reaction with Oximes Derived from Ketosteroids: Steroid-Pyrrole Structures. Russ. J. Org. Chem. 2003, 39, 1406–1411. [Google Scholar] [CrossRef]
- Koivukorpi, J.; Valkonen, A.; Kolehmainen, E. Synthesis and Characterization of Lithocholic Acid Derived Dipyrromethanes: Precursors for Pyrrole-Steroidal Macrocycles. J. Mol. Struct. 2004, 693, 81–86. [Google Scholar] [CrossRef]
- Shamsuzzaman; Siddiqui, T.; Alam, M.G.; Dar, A.M. Synthesis, Characterization and Anticancer Studies of New Steroidal Oxadiazole, Pyrrole and Pyrazole Derivatives. J. Saudi Chem. Soc. 2015, 19, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Metz, T.L.; Lutovsky, G.A.; Stanley, L.M. An Acid-Catalyzed Addition and Dehydration Sequence for the Synthesis of Heteroarylated Steroidal Dienes. J. Org. Chem. 2018, 83, 1643–1648. [Google Scholar] [CrossRef]
- Huong, N.T.T.; Klímková, P.; Sorrenti, A.; Mancini, G.; Drašar, P. Synthesis of Spiroannulated Oligopyrrole Macrocycles Derived from Lithocholic Acid. Steroids 2009, 74, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi, T.H.; Cardová, L.; Dvořáková, M.; Ročková, D.; Drašar, P. Synthesis of Cholic Acid Based Calixpyrroles and Porphyrins. Steroids 2012, 77, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Dukh, M.; Šaman, D.; Lang, K.; Pouzar, V.; Černý, I.; Drašar, P.; Král, V. Steroid—Porphyrin Conjugate for Saccharide Sensing in Protic Media. Org. Biomol. Chem. 2003, 1, 3458–3463. [Google Scholar] [CrossRef]
- Zhylitskaya, H.A.; Zhabinskii, V.N.; Litvinovskaya, R.P.; Lettieri, R.; Monti, D.; Venanzi, M.; Khripach, V.A.; Drašar, P. Design and Studies of Novel Polyoxysterol-Based Porphyrin Conjugates. Steroids 2012, 77, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Taba, F.; Sum, T.H.; Sintic, P.J.; Lundmark, A.H.; Crossley, M.J.; Taba, F.; Sum, T.H.; Sintic, P.J.; Lundmark, A.H.; Crossley, M.J. Synthesis of Steroid–Porphyrin Conjugates from Oestradiol, Oestrone, and Lithocholic Acid*. Aust. J. Chem. 2014, 67, 1632–1645. [Google Scholar] [CrossRef]
- Mueller, G.P.; Jiu, J. The Synthesis of Steroid Ring-E Pyrroles and Pyrrolidines. J. Org. Chem. 1961, 26, 1611–1614. [Google Scholar] [CrossRef]
- Alford, J.S.; Spangler, J.E.; Davies, H.M.L. Conversion of Cyclic Ketones to 2,3-Fused Pyrroles and Substituted Indoles. J. Am. Chem. Soc. 2013, 135, 11712–11715. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide Resistance Mechanisms in the Two-Spotted Spider Mite Tetranychus Urticae and Other Important Acari: A Review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Badawy, M.E.I.; Abdelgaleil, S.A.M.; Mahmoud, N.F.; Marei, A.E.-S.M. Preparation and Characterizations of Essential Oil and Monoterpene Nanoemulsions and Acaricidal Activity against Two-Spotted Spider Mite (Tetranychus Urticae Koch). Int. J. Acarol. 2018, 44, 330–340. [Google Scholar] [CrossRef]
- Collins, D.A. A Review of Alternatives to Organophosphorus Compounds for the Control of Storage Mites. J. Stored Prod. Res. 2006, 42, 395–426. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Stillatus, V.; Tirry, L. Genetic Analysis and Cross-Resistance Spectrum of a Laboratory-Selected Chlorfenapyr Resistant Strain of Two-Spotted Spider Mite (Acari: Tetranychidae). Exp. Appl. Acarol. 2004, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Dekeyser, M.A. Acaricide Mode of Action. Pest Manag. Sci. 2005, 61, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.; Van Pottelberge, S.; Tirry, L. Biochemical Analysis of a Chlorfenapyr-Selected Resistant Strain of Tetranychus Urticae Koch. Pest Manag. Sci. 2006, 62, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Zhang, P.-X.; Li, Y.-Q.; Song, H.-B.; Wang, Q.-M. Design, Synthesis, and Biological Evaluation of 2-Benzylpyrroles and 2-Benzoylpyrroles Based on Structures of Insecticidal Chlorfenapyr and Natural Pyrrolomycins. Mol. Divers. 2014, 18, 593–598. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Zhang, P.; Li, Y.; Xiong, L.; Wang, Q. Design, Synthesis, and Biological Evaluation of Various α-Substituted Benzylpyrroles Based on the Structures of Insecticidal Chlorfenapyr and Natural Pyrrolomycins. J. Agric. Food Chem. 2014, 62, 6072–6081. [Google Scholar] [CrossRef]
- Gould, F. Rapid Host Range Evolution in a Population of the Phytophagous Mite Tetranychus Urticae Koch. Evolution 1979, 33, 791–802. [Google Scholar] [CrossRef]
- Knowles, C.O. Mechanisms of Resistance to Acaricides. In Molecular Mechanisms of Resistance to Agrochemicals; Sjut, V., Ed.; Chemistry of Plant Protection; Springer: Berlin/Heidelberg, Germany, 1997; pp. 57–77. ISBN 978-3-662-03458-3. [Google Scholar]
- Akram, N.A.; Ashraf, M. Regulation in Plant Stress Tolerance by a Potential Plant Growth Regulator, 5-Aminolevulinic Acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Liu, C.; Yamamura, H.; Hayakawa, M.; Zhang, Z.; Oku, N.; Igarashi, Y. Plant Growth-Promoting and Antimicrobial Chloropyrroles from a Rare Actinomycete of the Genus Catellatospora. J. Antibiot. 2022, 75, 655–661. [Google Scholar] [CrossRef]
- Uhle, F.C.; Jacobs, W.A. The Veratrine Alkaloids: XXIV. The Octahydropyrrocoline Ring System of the Tertiary Bases. Conversion of Sarsasapogenin to a Solanidine Derivative. J. Biol. Chem. 1945, 160, 243–248. [Google Scholar] [CrossRef]
- Uhle, F.C.; Sallmann, F. The Synthesis and Transformations of a Steroid Pyrroline Derivative. J. Am. Chem. Soc. 1960, 82, 1190–1199. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-74168-9. [Google Scholar]
- Díaz-Ortiz, Á.; Prieto, P.; de la Hoz, A. A Critical Overview on the Effect of Microwave Irradiation in Organic Synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Panda, B. Microwave-Assisted Homogeneous Gold Catalyzed Organic Transformations. Curr. Microw. Chem. 2020, 7, 166–182. [Google Scholar] [CrossRef]
- Geetanjali; Singh, R. Microwave-Assisted Organic Synthesis in Water. Curr. Microw. Chem. 2021, 8, 117–127. [Google Scholar] [CrossRef]
- Corey, E.J.; Suggs, J.W. Pyridinium Chlorochromate. An Efficient Reagent for Oxidation of Primary and Secondary Alcohols to Carbonyl Compounds. Tetrahedron Lett. 1975, 16, 2647–2650. [Google Scholar] [CrossRef]
- Fernández-Herrera, M.A.; López-Muñoz, H.; Hernández-Vázquez, J.M.V.; López-Dávila, M.; Escobar-Sánchez, M.L.; Sánchez-Sánchez, L.; Pinto, B.M.; Sandoval-Ramírez, J. Synthesis of 26-Hydroxy-22-Oxocholestanic Frameworks from Diosgenin and Hecogenin and Their in Vitro Antiproliferative and Apoptotic Activity on Human Cervical Cancer CaSki Cells. Bioorg. Med. Chem. 2010, 18, 2474–2484. [Google Scholar] [CrossRef] [PubMed]
- Zeferino-Díaz, R.; Olivera-Castillo, L.; Dávalos, A.; Grant, G.; Kantún-Moreno, N.; Rodriguez-Canul, R.; Bernès, S.; Sandoval-Ramírez, J.; Fernández-Herrera, M.A. 22-Oxocholestane Oximes as Potential Anti-Inflammatory Drug Candidates. Eur. J. Med. Chem. 2019, 168, 78–86. [Google Scholar] [CrossRef]
- Tiwari, B.; Zhang, J.; Chi, Y.R. Facile Access to Chiral Ketones through Metal-Free Oxidative C-C Bond Cleavage of Aldehydes by O2. Angew. Chem. 2012, 124, 1947–1950. [Google Scholar] [CrossRef]
- Hamid, A.A.; Hasanain, M.; Singh, A.; Bhukya, B.; Omprakash; Vasudev, P.G.; Sarkar, J.; Chanda, D.; Khan, F.; Aiyelaagbe, O.O.; et al. Synthesis of Novel Anticancer Agents through Opening of Spiroacetal Ring of Diosgenin. Steroids 2014, 87, 108–118. [Google Scholar] [CrossRef]
- Ngufor, C.; Critchley, J.; Fagbohoun, J.; N’Guessan, R.; Todjinou, D.; Rowland, M. Chlorfenapyr (A Pyrrole Insecticide) Applied Alone or as a Mixture with Alpha-Cypermethrin for Indoor Residual Spraying against Pyrethroid Resistant Anopheles Gambiae Sl: An Experimental Hut Study in Cove, Benin. PLoS ONE 2016, 11, e0162210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Chauhan, U.; Kumari, A.; Nadda, G. Comparative Toxicities of Novel and Conventional Acaricides against Different Stages of Tetranychus Urticae Koch (Acarina: Tetranychidae). J. Saudi Soc. Agric. Sci. 2017, 16, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R.; Kemp, K.E. Evaluation of Persistence of Selected Miticides Against the Twospotted Spider Mite, Tetranychus Urticae. HortScience 2009, 44, 476–480. [Google Scholar] [CrossRef]
- Pacifici, E.; Polverari, L.; Sabatini, S. Plant Hormone Cross-Talk: The Pivot of Root Growth. J. Exp. Bot. 2015, 66, 1113–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestrelab Research chemistry software solutions. Analytical Chemistry Software Solutions. Available online: https://mestrelab.com/ (accessed on 17 November 2022).
- Zeferino-Diaz, R.; Hilario-Martinez, J.C.; Rodriguez-Acosta, M.; Sandoval-Ramirez, J.; Fernandez-Herrera, M.A. 22-Oxocholestanes as Plant Growth Promoters. Steroids 2015, 98, 126–131. [Google Scholar] [CrossRef]
- Zeferino-Diaz, R.; Hilario-Martinez, J.C.; Rodriguez-Acosta, M.; Carrasco-Carballo, A.; Hernandez-Linares, M.G.; Sandoval-Ramirez, J.; Fernandez-Herrera, M.A. Mimicking Natural Phytohormones. 26-Hydroxycholestan-22-One Derivatives as Plant Growth Promoters. Steroids 2017, 125, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, S.C.H.; Niculau, E.D.S.; Blank, A.F.; Câmara, C.A.G.; Araújo, I.N.; Alves, P.B. Composition and Acaricidal Activity of Lippia Sidoides Essential Oil against Two-Spotted Spider Mite (Tetranychus Urticae Koch). Bioresour. Technol. 2010, 101, 829–832. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Arami, S.A.A.; Abdelgaleil, S.A.M. Acaricidal and Quantitative Structure Activity Relationship of Monoterpenes against the Two-Spotted Spider Mite, Tetranychus Urticae. Exp. Appl. Acarol. 2010, 52, 261–274. [Google Scholar] [CrossRef]
- Cua-Basulto, M.E.; Ruiz-Sánchez, E.; Pérez-Gutiérrez, A.; Martín-Mex, R.; Nexticapan-Garcéz, Á.; Pérez-Brito, D. Effects of Acaricides on Oligonychus Sp. and Compatibility with Predatory Mites Neoseiulus Californicus and Phytoseiulus Persimilis. J. Plant Dis. Prot. 2021, 128, 1617–1625. [Google Scholar] [CrossRef]




| Entry | Solvent [a] | Reaction Time (h) [b] | Yield of 3a (%) [c] |
|---|---|---|---|
| 1 | acetonitrile | 6 | 38 |
| 2 | ethanol | 6 | 45 |
| 3 | toluene | 5 | 80 |
| 4 | xylene | 5 | 65 |
| 5 | DMF | 6 | 32 |
| Position | 3a | 3b | 3c | 8a | 8b | 8c |
|---|---|---|---|---|---|---|
| 3 | 4.59 | 4.60 | 4.60 | 4.60 | 4.60 | 4.59 |
| 6 | 5.36 | 5.39 | 5.40 | 5.36 | 5.36 | 5.36 |
| 16 | - | - | - | 4.97 | 4.98 | 4.95 |
| 18 | 0.91 | 0.87 | 0.88 | 0.90 | 0.95 | 0.90 |
| 19 | 1.07 | 1.07 | 1.08 | 1.02 | 1.04 | 1.03 |
| 20 | - | - | - | 2.99 | 3.06 | 3.05 |
| 21 | 1.98 | 1.95 | 1.96 | 0.84 | 1.22 | 1.23 |
| 23, 24 | - | - | - | 5.85 | 5.78 | 5.75 |
| 26 | 3.82, 3.77 | 3.94 | 3.98, 3.93 | 2.09 | 2.20 | 2.18 |
| 27 | 0.84 | 0.98 | 1.00 | - | - | - |
| Position | 3a | 3b | 3c | 8a | 8b | 8c |
|---|---|---|---|---|---|---|
| 15 | 22.2 | 21.9 | 22.0 | 34.8 | 35.0 | 34.9 |
| 16 | 129.7 | 129.8 | 129.3 | 76.1 | 76.5 | 76.2 |
| 17 | 135.0 | 135.2 | 135.0 | 60.4 | 59.4 | 60.3 |
| 18 | 18.5 | 18.3 | 18.3 | 12.7 | 12.9 | 12.8 |
| 19 | 19.3 | 19.2 | 19.2 | 19.3 | 19.3 | 19.3 |
| 20 | 109.3 | 109.1 | 108.8 | 28.2 | 28.2 | 28.9 |
| 21 | 10.0 | 9.8 | 9.9 | 22.6 | 23.4 | 23.0 |
| 22 | 134.2 | 133.8 | 133.3 | 138.0 | 137.7 | 137.3 |
| 23 | 26.1 | 26.4 | 26.4 | 102.8 | 102.7 | 102.3 |
| 24 | 34.2 | 34.3 | 34.1 | 106.1 | 106.2 | 105.9 |
| 25 | 32.4 | 32.6 | 32.6 | 126.3 | 126.5 | 125.6 |
| 26 | 69.0 | 68.9 | 69.0 | 12.4 | 12.7 | 12.4 |
| 27 | 16.7 | 16.8 | 16.8 | - | - | - |
| Compound | % Mortality of Adults | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | % Mortality of Eggs | |
| 8a | 11.3 ± 2.4 | 16.6 ± 1.1 | 31.3 ± 2.6 | 26.25 ± 3.9 |
| 8b | 9.3 ± 1.4 | 19.3 ± 3.0 | 26.0 ± 2.3 | 48.75 ± 4.7 |
| 8c | 5.3 ± 1.6 | 17.3 ± 1.7 | 39.3 ± 2.7 | 56.87 ± 3.12 |
| Control | 2.0 ± 1.0 | 11.3 ± 2.2 | 23.3 ± 2.4 | 14.37 ± 5.38 |
| Compound | Plant Height (cm) | Number of Leaves/Plant | Stem Dry Biomass (g) | Leaves Dry Biomass (g) | Root Dry Biomass (g) |
|---|---|---|---|---|---|
| 8a | 13.5 ± 0.51 | 9.0 ± 0.16 | 0.1 ± 0.00 | 0.37 ± 0.01 | 0.10 ± 0.00 |
| 8b | 13.2 ± 0.44 | 8.6 ± 0.27 | 0.1 ± 0.00 | 0.31 ± 0.04 | 0.12 ± 0.01 |
| 8c | 12.7 ± 0.28 | 8.6 ± 0.13 | 0.1 ± 0.00 | 0.35 ± 0.02 | 0.15 ± 0.02 |
| Control * | 13.2 ± 0.40 | 8.6 ± 0.18 | 0.1 ± 0.00 | 0.34 ± 0.02 | 0.10 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De los Santos, M.G.; Cua-Basulto, M.; Huepalcalco, A.; Delit, W.; Sandoval-Ramírez, J.; López-Torres, A.; Ruiz-Sánchez, E.; Fernández-Herrera, M.A. Fused Pyrroles in Cholestane and Norcholestane Side Chains: Acaricidal and Plant Growth-Promoting Effects. Molecules 2022, 27, 8466. https://doi.org/10.3390/molecules27238466
De los Santos MG, Cua-Basulto M, Huepalcalco A, Delit W, Sandoval-Ramírez J, López-Torres A, Ruiz-Sánchez E, Fernández-Herrera MA. Fused Pyrroles in Cholestane and Norcholestane Side Chains: Acaricidal and Plant Growth-Promoting Effects. Molecules. 2022; 27(23):8466. https://doi.org/10.3390/molecules27238466
Chicago/Turabian StyleDe los Santos, María G., Marcos Cua-Basulto, Anallely Huepalcalco, Wendy Delit, Jesús Sandoval-Ramírez, Adolfo López-Torres, Esaú Ruiz-Sánchez, and María A. Fernández-Herrera. 2022. "Fused Pyrroles in Cholestane and Norcholestane Side Chains: Acaricidal and Plant Growth-Promoting Effects" Molecules 27, no. 23: 8466. https://doi.org/10.3390/molecules27238466
APA StyleDe los Santos, M. G., Cua-Basulto, M., Huepalcalco, A., Delit, W., Sandoval-Ramírez, J., López-Torres, A., Ruiz-Sánchez, E., & Fernández-Herrera, M. A. (2022). Fused Pyrroles in Cholestane and Norcholestane Side Chains: Acaricidal and Plant Growth-Promoting Effects. Molecules, 27(23), 8466. https://doi.org/10.3390/molecules27238466