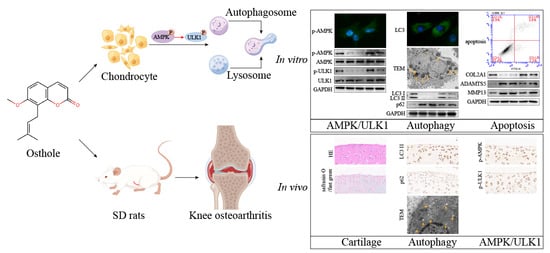
Osthole Suppresses Knee Osteoarthritis Development by Enhancing Autophagy Activated via the AMPK/ULK1 Pathway
Abstract
:1. Introduction
2. Results
2.1. Osthole Rescues Chondrocytes Treated with IL-1β
2.2. Osthole Delays OA-Related Degeneration in IL-1β-Treated Chondrocytes
2.3. Osthole Attenuates IL-1β-Induced Chondrocyte Degeneration by Activating Autophagy
2.4. Autophagy Is Promoted by Phosphorylating AMPK/ULK1 in Osthole-Treated Chondrocytes
2.5. Osthole Suppresses KOA Progression in a Dose-Dependent Manner In Vivo
2.6. High Dose Osthole Phosphorylates AMPK/ULK1 and Activates Autophagy In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Culture and Identification of Primary Chondrocytes from SD Rats
4.3. Cell Treatment
4.4. Cell Viability Assay
4.5. Apoptosis Detection
4.6. Transmission Electron Microscopy (TEM)
4.7. Immunofluorescence Staining
4.8. Western Blotting
4.9. Quantitative Real-Time Polymerase Chain Reaction Analysis (qPCR)
4.10. Animals
4.11. Histology and IHC Analysis
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Lin, Z.; Miao, J.; Zhang, T.; He, M.; Zhou, X.; Zhang, H.; Gao, Y.; Bai, L. d-Mannose suppresses osteoarthritis development in vivo and delays IL-1beta-induced degeneration in vitro by enhancing autophagy activated via the AMPK pathway. Biomed Pharmacother. 2021, 135, 111199. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Li, Q.; Yang, L.; Zhang, Y.; Qiu, D. The Use of 1H-qNMR Method for Simultaneous Determination of Osthol, Columbianadin, and Isoimperatorin in Angelicae Pubescentis Radix. J. AOAC Int. 2020, 103, 851–856. [Google Scholar] [CrossRef]
- Hua, K.F.; Yang, S.M.; Kao, T.Y.; Chang, J.M.; Chen, H.L.; Tsai, Y.J.; Chen, A.; Yang, S.S.; Chao, L.K.; Ka, S.M. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and NF-kappaB/NLRP3 pathway. PLoS ONE 2013, 8, e77794. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhu, D.; Liu, P.; Yang, Q.; Gao, J.; Huang, Y.; Chen, Y.; Gao, Y.; Zhang, C. Osthole stimulates bone formation, drives vascularization and retards adipogenesis to alleviate alcohol-induced osteonecrosis of the femoral head. J. Cell Mol. Med. 2020, 24, 4439–4451. [Google Scholar] [CrossRef] [Green Version]
- Chern, C.M.; Zhou, H.; Wang, Y.H.; Chang, C.L.; Chiou, W.F.; Chang, W.T.; Yao, C.H.; Liou, K.T.; Shen, Y.C. Osthole ameliorates cartilage degradation by downregulation of NF-kappaB and HIF-2alpha pathways in an osteoarthritis murine model. Eur. J. Pharmacol. 2020, 867, 172799. [Google Scholar] [CrossRef]
- Liu, L.; Xu, L.; Wang, S.; Wang, L.; Wang, X.; Xu, H.; Li, X.; Ye, H. Confirmation of inhibitingTLR4/MyD88/NF-kappaB Signalling Pathway by Duhuo Jisheng Decoction on Osteoarthritis: A Network Pharmacology Approach-Integrated Experimental Study. Front. Pharmacol. 2021, 12, 784822. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhao, M.; Zhang, P.; Yang, L.S.; Bi, R.X.; Xie, W.P. Cangxitongbi capsules protect the articular cartilage in the rat knee through the long non-coding RNA HOTAIR/p38MAPK pathway. Ann. Transl. Med. 2022, 10, 23. [Google Scholar] [CrossRef]
- Song, X.Y.; Xie, W.P.; Zhang, P.; Zhao, M.; Bi, R.X. Cangxitongbi capsule protects articular cartilage of the knee in rats by regulating ADAMTS-5. Ann. Transl. Med. 2020, 8, 1511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, B.; Liu, W.X.; Lu, K.; Pan, H.; Wang, T.; Oh, C.D.; Yi, D.; Huang, J.; Zhao, L.; et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 2020, 79, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Hua, R.; Ma, J.; Zhou, Y.; Li, P.; Xu, X.; Yu, Z.; Quan, S. Bisphenol A promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ. Int. 2021, 147, 106298. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Xie, H.; Liu, Z.Z. The Role of Autophagy in Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 608388. [Google Scholar] [CrossRef]
- Jeon, H.; Im, G.I. Autophagy in osteoarthritis. Connect. Tissue Res. 2017, 58, 497–508. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Yu, Y.; Jia, L.; Song, X.; Xu, X.; Li, T.; Sheng, X.; Wang, H.; Zhang, J.; et al. Bilobalide Exerts Anti-Inflammatory Effects on Chondrocytes Through the AMPK/SIRT1/mTOR Pathway to Attenuate ACLT-Induced Post-Traumatic Osteoarthritis in Rats. Front. Pharmacol. 2022, 13, 783506. [Google Scholar] [CrossRef]
- Pei, W.; Huang, X.; Ni, B.; Zhang, R.; Niu, G.; You, H. Selective STAT3 Inhibitor Alantolactone Ameliorates Osteoarthritis via Regulating Chondrocyte Autophagy and Cartilage Homeostasis. Front. Pharmacol. 2021, 12, 730312. [Google Scholar] [CrossRef]
- Pan, X.; Shan, H.; Bai, J.; Gao, T.; Chen, B.; Shen, Z.; Zhou, H.; Lu, H.; Sheng, L.; Zhou, X. Four-octyl itaconate improves osteoarthritis by enhancing autophagy in chondrocytes via PI3K/AKT/mTOR signalling pathway inhibition. Commun. Biol. 2022, 5, 641. [Google Scholar] [CrossRef]
- Abramson, S.B.; Attur, M. Developments in the scientific understanding of osteoarthritis. Arthritis Res. Ther. 2009, 11, 227. [Google Scholar] [CrossRef]
- Stanton, H.; Rogerson, F.; East, C.; Golub, S.; Lawlor, K.; Meeker, C.; Little, C.; Last, K.; Farmer, P.; Campbell, I.; et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005, 434, 648–652. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Shi, J.; Liu, L.; Ma, H.; He, L.; Guo, Y. Downregulation of miRNA-30a enhanced autophagy in osthole-alleviated myocardium ischemia/reperfusion injury. J. Cell. Physiol 2019, 1–10. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Wang, X.; Wang, J.; Hao, Q.; Hao, J.; Hou, X. Osthole-Loaded Nanoemulsion Enhances Brain Target in the Treatment of Alzheimer’s Disease via Intranasal Administration. Oxid. Med. Cell. Longev. 2021, 2021, 8844455. [Google Scholar] [CrossRef]
- Pujol, J.P.; Chadjichristos, C.; Legendre, F.; Bauge, C.; Beauchef, G.; Andriamanalijaona, R.; Galera, P.; Boumediene, K. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect. Tissue Res. 2008, 49, 293–297. [Google Scholar] [CrossRef]
- Gun Bilgic, D.; Hatipoglu, O.F.; Cigdem, S.; Bilgic, A.; Cora, T. NF-kbeta upregulates ADAMTS5 expression by direct binding after TNF-alpha treatment in OUMS-27 chondrosarcoma cell line. Mol. Biol. Rep. 2020, 47, 4215–4223. [Google Scholar] [CrossRef]
- Bokhari, R.A.; Tantowi, N.; Lau, S.F.; Mohamed, S. Java Tea (Orthosiphon stamineus) protected against osteoarthritis by mitigating inflammation and cartilage degradation: A preclinical study. Inflammopharmacology 2018, 26, 939–949. [Google Scholar] [CrossRef]
- Guo, Y.F.; Su, T.; Yang, M.; Li, C.J.; Guo, Q.; Xiao, Y.; Huang, Y.; Liu, Y.; Luo, X.H. The role of autophagy in bone homeostasis. J. Cell. Physiol. 2021, 236, 4152–4173. [Google Scholar] [CrossRef]
- Pierrefite-Carle, V.; Santucci-Darmanin, S.; Breuil, V.; Camuzard, O.; Carle, G.F. Autophagy in bone: Self-eating to stay in balance. Ageing Res. Rev. 2015, 24, 206–217. [Google Scholar] [CrossRef]
- Narendra, D.; Kane, L.A.; Hauser, D.N.; Fearnley, I.M.; Youle, R.J. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 2010, 6, 1090–1106. [Google Scholar] [CrossRef]
- Xin, R.; Xu, Y.; Long, D.; Mao, G.; Liao, H.; Zhang, Z.; Kang, Y. Mitochonic Acid-5 Inhibits Reactive Oxygen Species Production and Improves Human Chondrocyte Survival by Upregulating SIRT3-Mediated, Parkin-dependent Mitophagy. Front. Pharmacol. 2022, 13, 911716. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, H.; Zhang, X.; Xu, Y.; Fu, Z.; Ji, X.; Wei, C.; An, G.; Tan, M.; Zhou, M. Columbianetin alleviates lipopolysaccharides (LPS)-induced inflammation and apoptosis in chondrocyte through activation of autophagy by inhibiting serum and glucocorticoid-induced protein kinase 1 (SGK1) expression. Bioengineered 2022, 13, 4051–4062. [Google Scholar] [CrossRef]
- He, R.; Wang, Z.; Cui, M.; Liu, S.; Wu, W.; Chen, M.; Wu, Y.; Qu, Y.; Lin, H.; Chen, S.; et al. HIF1A Alleviates compression-induced apoptosis of nucleus pulposus derived stem cells via upregulating autophagy. Autophagy 2021, 17, 3338–3360. [Google Scholar] [CrossRef]
- Li, Q.; Yue, Y.; Chen, L.; Xu, C.; Wang, Y.; Du, L.; Xue, X.; Liu, Q.; Wang, Y.; Fan, F. Resveratrol Sensitizes Carfilzomib-Induced Apoptosis via Promoting Oxidative Stress in Multiple Myeloma Cells. Front. Pharmacol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Liu, Y.; Huang, C.; Xia, W.; Zhou, H.; Zhou, Z.; Zhou, X. Luteolin Protects Chondrocytes from H2O2-Induced Oxidative Injury and Attenuates Osteoarthritis Progression by Activating AMPK-Nrf2 Signaling. Oxid. Med. Cell. Longev. 2022, 2022, 5635797. [Google Scholar] [CrossRef]
- Tamargo-Gomez, I.; Marino, G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018, 19, 3812. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Li, W.; Chen, Y.; Yan, Z.; Huang, X.; Zhuang, H.; Zhong, W.; Chen, Y.; Wu, W.; Lin, C.; et al. Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS Lett. 2015, 589, 1847–1854. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, L.; Yang, Y.; Zheng, H.; Cai, Y.; Ma, Y.; Gu, R.; Xu, K.; Zhang, R.; Xu, P. Metformin Ameliorates Senescence of Adipose-Derived Mesenchymal Stem Cells and Attenuates Osteoarthritis Progression via the AMPK-Dependent Autophagy Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 4620254. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, C.; Liu, P.; Huang, H.; Zhang, S.; Wang, X. Anti-Apoptosis and Autophagy Effects of Melatonin Protect Rat Chondrocytes against Oxidative Stress via Regulation of AMPK/Foxo3 Pathways. Cartilage 2021, 13, 1041S–1053S. [Google Scholar] [CrossRef]
- Hu, J.; Cui, W.; Ding, W.; Gu, Y.; Wang, Z.; Fan, W. Globular Adiponectin Attenuated H2O2-Induced Apoptosis in Rat Chondrocytes by Inducing Autophagy Through the AMPK/ mTOR Pathway. Cell. Physiol. Biochem. 2017, 43, 367–382. [Google Scholar] [CrossRef]
- Wang, X.P.; Xie, W.P.; Bi, Y.F.; Wang, B.A.; Song, H.B.; Wang, S.L.; Bi, R.X. Quercetin suppresses apoptosis of chondrocytes induced by IL-1beta via inactivation of p38 MAPK signaling pathway. Exp. Ther. Med. 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Khairy, H.; Saleh, H.; Badr, A.M.; Marie, M.S. Therapeutic efficacy of osthole against dinitrobenzene sulphonic acid induced-colitis in rats. Biomed. Pharmacother. 2018, 100, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Z.; Hou, W.; Zhou, Q.; Zhang, M.; Holz, J.; Sheu, T.J.; Li, T.F.; Cheng, S.D.; Shi, Q.; Harris, S.E.; et al. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J. Bone Miner. Res. 2010, 25, 1234–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, H.; Shimizu, M.; Tsuji, H. Cartilage and subchondral bone interaction in osteoarthrosis of human knee joint: A histological and histomorphometric study. Microsc. Res. Tech. 1997, 37, 333–342. [Google Scholar] [CrossRef]







| Name | Primer | Sequence |
|---|---|---|
| COL2A1 | Forward Reverse | GACGCCACGCTCAGTC TCTCCGCTCTTCCACTCTG |
| ADAMTS5 | Forward Reverse | GCATTACCTGCTGACCCT TTCTTGCTCACCTCCAGAC |
| MMP13 | Forward Reverse | TGGGCCTTCTGGTCTTC GTTGTAGCCTTTGGAGATG |
| LC3A/B | Forward Reverse | TGCACTCGCCTTGTACG CTCTTCCGTTGCTGTTGC |
| P62 | Forward Reverse | GACTTGGTCGCCTTCTCC ATGCTTCGTGCCTCCTG |
| GAPDH | Forward Reverse | CCTTCCGTGTCCCCACT GCCTGCTTCACCACCTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Wang, X.; Qu, W.; Yang, L.; Jing, C.; Zhu, B.; Zhang, Y.; Xie, W. Osthole Suppresses Knee Osteoarthritis Development by Enhancing Autophagy Activated via the AMPK/ULK1 Pathway. Molecules 2022, 27, 8624. https://doi.org/10.3390/molecules27238624
Ma T, Wang X, Qu W, Yang L, Jing C, Zhu B, Zhang Y, Xie W. Osthole Suppresses Knee Osteoarthritis Development by Enhancing Autophagy Activated via the AMPK/ULK1 Pathway. Molecules. 2022; 27(23):8624. https://doi.org/10.3390/molecules27238624
Chicago/Turabian StyleMa, Teng, Xiangpeng Wang, Wenjing Qu, Lingsen Yang, Cheng Jing, Bingrui Zhu, Yongkui Zhang, and Wenpeng Xie. 2022. "Osthole Suppresses Knee Osteoarthritis Development by Enhancing Autophagy Activated via the AMPK/ULK1 Pathway" Molecules 27, no. 23: 8624. https://doi.org/10.3390/molecules27238624
APA StyleMa, T., Wang, X., Qu, W., Yang, L., Jing, C., Zhu, B., Zhang, Y., & Xie, W. (2022). Osthole Suppresses Knee Osteoarthritis Development by Enhancing Autophagy Activated via the AMPK/ULK1 Pathway. Molecules, 27(23), 8624. https://doi.org/10.3390/molecules27238624