Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
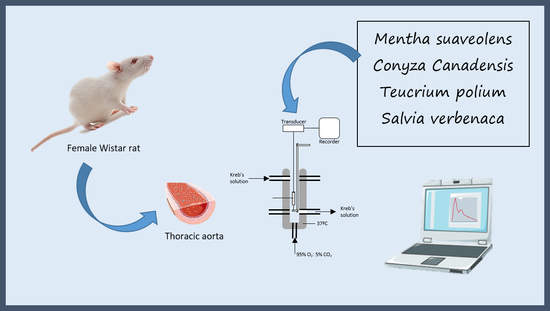
4.1. Chemicals and Preparation of Solutions
4.2. Plant Materials
4.3. Preparations of the Extracts
4.4. Phytochemical Screening
4.5. Phenolic Compounds Analysis
4.6. Functional Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, T.; Zemlin, A.E.; Erasmus, R.T.; Madlala, S.S.; Faber, M.; Kengne, A.P. Assessment of the association between plant-based dietary exposures and cardiovascular disease risk profile in sub-Saharan Africa: A systematic review. BMC Public Health 2022, 22, 361. [Google Scholar] [CrossRef]
- Chavez-Castillo, M.; Ortega, A.; Duran, P.; Pirela, D.; Marquina, M.; Cano, C.; Salazar, J.; Gonzalez, M.C.; Bermudez, V.; Rojas-Quintero, J.; et al. Phytotherapy for Cardiovascular Disease: A Bench-to-Bedside Approach. Curr. Pharm. Des. 2020, 26, 4410–4429. [Google Scholar] [CrossRef] [PubMed]
- Kamyab, R.; Namdar, H.; Torbati, M.; Ghojazadeh, M.; Araj-Khodaei, M.; Fazljou, S.M.B. Medicinal Plants in the Treatment of Hypertension: A Review. Adv. Pharm. Bull. 2021, 11, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Sinha, M.; Bansal, N.; Yadav, S.R.; Shah, K.; Chauhan, N.S. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2021, 11, 155–184. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Kim, B.; Lee, K.; Chinannai, K.S.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.Y. Endothelium-Independent Vasorelaxant Effect of Ligusticum jeholense Root and Rhizoma on Rat Thoracic Aorta. Molecules 2015, 20, 10721–10733. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as Factories for Human Pharmaceuticals: Applications and Challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef]
- Hao, D.C.; Xiao, P.G. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Bozovic, M.; Pirolli, A.; Ragno, R. Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules 2015, 20, 8605–8633. [Google Scholar] [CrossRef]
- Bello, R.; Calatayud, S.; Beltran, B.; Primo-Yufera, E.; Esplugues, J. Cardiovascular effects of the methanol and dichloromethanol extracts from Mentha suaveolens Ehrh. Phytother. Res. 2001, 15, 447–448. [Google Scholar] [CrossRef]
- Guedes, D.N.; Silva, D.F.; Barbosa-Filho, J.M.; Medeiros, I.A. Muscarinic agonist properties involved in the hypotensive and vasorelaxant responses of rotundifolone in rats. Planta Med. 2002, 68, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Kasrati, A.; Alaoui Jamali, C.; Bekkouche, K.; Spooner-Hart, R.; Leach, D.; Abbad, A. Chemical characterization and insecticidal properties of essential oils from different wild populations of Mentha suaveolens subsp. timija (Briq.) Harley from Morocco. Chem. Biodivers. 2015, 12, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kasrati, A.; Jamali, C.A.; Bekkouche, K.; Lahcen, H.; Markouk, M.; Wohlmuth, H.; Leach, D.; Abbad, A. Essential oil composition and antimicrobial activity of wild and cultivated mint timija (Mentha suaveolens subsp. timija (Briq.) Harley), an endemic and threatened medicinal species in Morocco. Nat. Prod. Res. 2013, 27, 1119–1122. [Google Scholar] [CrossRef]
- Healthcare, T. PDR for Herbal Medicines, 4th ed.; Thomson: Belmont, CA, USA, 2008. [Google Scholar]
- Veres, K.; Csupor-Loffler, B.; Lazar, A.; Hohmann, J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. Sci. World J. 2012, 2012, 489646. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, S.A.; Ondua, M.; Shai, L.J.; Lebelo, S.L. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. J. Inflamm. Res. 2019, 12, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrabti, H.N.; El Menyiy, N.; Charfi, S.; Saber, M.; Bakrim, S.; Alyamani, R.A.; Rauf, A.; Ali, A.M.H.; Abdallah, E.M.; El Omari, N.; et al. Phytochemistry and Biological Properties of Salvia verbenaca L.: A Comprehensive Review. Biomed Res. Int. 2022, 2022, 3787818. [Google Scholar] [CrossRef]
- Orch, H.; Douira, A.; Zidane, L. Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète, et des maladies cardiaques dans la région d’Izarène (Nord du Maroc). J. Appl. Biosci. 2015, 86, 7940–7956. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Chaouch-Hamada, R.; Sotomayor, J.A.; Landoulsi, A.; Jordan, M.J. Antioxidant properties and evaluation of phytochemical composition of Salvia verbenaca L. extracts at different developmental stages. Plant Foods Hum. Nutr. 2015, 70, 15–20. [Google Scholar] [CrossRef]
- Canzoneri, M.; Bruno, M.; Rosselli, S.; Russo, A.; Cardile, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and biological activity of Salvia verbenaca essential oil. Nat. Prod. Commun. 2011, 6, 1023–1026. [Google Scholar] [CrossRef]
- Bahramikia, S.; Yazdanparast, R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae). Phytother. Res. 2012, 26, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.S.; Abdul-Ghani, A.S.; Al-Khalil, S.; Amin, R. Effect of Teucrium polium boiled leaf extract on intestinal motility and blood pressure. J. Ethnopharmacol. 1988, 22, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudabady, M.; Shafei, M.N.; Niazmand, S.; Khodaee, E. The Effects of Hydroalchoholic Extract of Teucrium polium L. on Hypertension Induced by Angiotensin II in Rats. Int. J. Prev. Med. 2014, 5, 1255–1260. [Google Scholar] [PubMed]
- Purnavab, S.; Ketabchi, S.; Rowshan, V. Chemical composition and antibacterial activity of methanolic extract and essential oil of Iranian Teucrium polium against some of phytobacteria. Nat. Prod. Res. 2015, 29, 1376–1379. [Google Scholar] [CrossRef]
- Nikpour, H.; Mousavi, M.; Asadollahzadeh, H. Qualitative and quantitative analysis of Teucrium polium essential oil components by GC-MS coupled with MCR and PARAFAC methods. Phytochem. Anal. 2018, 29, 590–600. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Ferreres, F.; Bernardo, J.; Andrade, P.B.; Sousa, C.; Gil-Izquierdoa, A.; Valentão, P. Pennyroyal and gastrointestinal cells: Multi-target protection of phenolic compounds against t-BHP-induced toxicity. RSC Adv. 2015, 5, 41576–41584. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Alegría-Herrera, E.; Herrera-Ruiz, M.; Román-Ramos, R.; Zamilpa, A.; Santillán-Urquiza, M.A.; Aguilar, M.I.; Avilés-Flores, M.; Fuentes-Mata, M.; Jiménez-Ferrer, E. Effect of Ocimumbasilicum, Ocimumselloi, and Rosmarinic Acid on Cerebral Vascular Damage in a Chronic Hypertension Model. Biol. Pharm. Bull. 2019, 42, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.G.; Evora, P.R.B.; Capellini, V.K.; Albuquerque, A.A.; Carvalho, M.T.M.; Gomes, R.; Parolini, M.T.; Celotto, A.C. Effect of rosmarinic acid on the arterial blood pressure in normotensive and hypertensive rats: Role of ACE. Phytomedicine 2018, 38, 158–165. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, B.; Xu, B.; Mi, X.; Li, G.; Ma, C.; Xie, J.; Li, J.; Wang, Z. Rosmarinic Acid Alleviates the Endothelial Dysfunction Induced by Hydrogen Peroxide in Rat Aortic Rings via Activation of AMPK. Oxidative Med. Cell Longev. 2017, 2017, 7091904. [Google Scholar] [CrossRef] [Green Version]
- Hugel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol protection and treatment of hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Harvey, R.D. Muscarinic receptor agonists and antagonists: Effects on cardiovascular function. Handb. Exp. Pharmacol. 2012, 208, 299–316. [Google Scholar] [CrossRef]
- Cuthbert, A.W. Some Effects of Atropine on Smooth Muscle. Br. J. Pharmacol. Chemother. 1963, 21, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Calver, A.; Collier, J.; Vallance, P. Nitric oxide and cardiovascular control. Exp. Physiol. 1993, 78, 303–326. [Google Scholar]
- Rapoport, R.M.; Murad, F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ. Res. 1983, 52, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Herald, A.K.; Touyz, R.M. Androgens and Androgen Receptors as Determinants of Vascular Sex Differences Across the Lifespan. Can. J. Cardiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Riedel, K.; Deussen, A.J.; Tolkmitt, J.; Weber, S.; Schlinkert, P.; Zatschler, B.; Friebel, C.; Muller, B.; El-Armouche, A.; Morawietz, H.; et al. Estrogen determines sex differences in adrenergic vessel tone by regulation of endothelial beta-adrenoceptor expression. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H243–H254. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J. Pharmacognosie, Phytochimie, Plants Médicinals, 5th ed.; Doc, É.T., Ed.; Technique et Documentation, Lavoisier: Paris, France, 2016. [Google Scholar]
- Oliveira, A.P.; Valentao, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and biological screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef] [PubMed]




| Samples | Phenolic Compounds | Tannins | Alkaloids |
|---|---|---|---|
| MS | + | − | − |
| SV | + | − | − |
| TP | + | − | − |
| CC | + | + | − |
| Phenolics | mg/g of Dry Extract | ||||
|---|---|---|---|---|---|
| MS | CC | TP | SV | ||
| 1 | 3-O-CQA | - | 0.30 ± 0.00 (2.51) | 0.11 ± 0.00 (91.67) | 0.10 ± 0.01 (25.64) |
| 2 | 4-O-CQA | 2.13 ± 0.04 (8.22) | 0.20 ± 0.00 (1.67) | - | 0.18 ± 0.00 (46.15) |
| 3 | 5-O-CQA | 3.97 ± 0.20 (15.33) | 2.68 ± 0.02 (22.41) | - | - |
| 4 | Isoferulicacid | - | - | 0.01 ± 0.00 (8.33) | - |
| 5 | 3,4-di-O-CQA | - | 0.54 ± 0.01 (4.52) | - | - |
| 6 | 3,5-di-O-CQA | - | 4.17 ± 0.19 (34.87) | - | - |
| 7 | 1,5-di-O-CQA | - | 0.76 ± 0.04 (6.35) | - | - |
| 8 | Rosmarinic acid | 19.80 ± 1.08 (76.45) | - | - | 0.11 ± 0.00 (28.21) |
| 9 | 4,5-di-O-CQA | - | 3.31 ± 0.28 (27.68) | - | - |
| Total | 25.90 | 11.96 | 0.12 | 0.39 | |
| Emax (% NA) | Control | L-NAME | Atropine | Indomethacin |
|---|---|---|---|---|
| MS-Lower | 40.26 ± 6.75 | 7.50 ± 3.38 * | 5.20 ± 2.22 * | 41.50 ± 11.50 |
| MS-Higher | 101.40 ± 14.67 | 114.00 ± 25.77 | 79.16 ± 2.44 | 75.20 ± 19.09 |
| SV-Lower | 35.44 ± 4.37 | 14.46 ± 6.78 | 22.89 ± 9.97 * | 2.98 ± 10.46 * |
| SV-Higher | 94.95 ± 2.40 | 114.50 ± 12.18 | 73.61 ± 15.97 | 70.30 ± 14.61 |
| TP | 122.1 ± 8.80 | 143.80 ± 17.72 | 106.10 ± 5.90 | 123.00 ± 18.61 |
| CC | 126.70 ± 19.11 | 154.30 ± 25.31 | 123.20 ± 13.37 | 119.00 ± 20.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Akhal, J.; Oliveira, A.P.; Bencheikh, R.; Valentão, P.; Andrade, P.B.; Morato, M. Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats. Molecules 2022, 27, 8752. https://doi.org/10.3390/molecules27248752
El-Akhal J, Oliveira AP, Bencheikh R, Valentão P, Andrade PB, Morato M. Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats. Molecules. 2022; 27(24):8752. https://doi.org/10.3390/molecules27248752
Chicago/Turabian StyleEl-Akhal, Jamila, Andreia P. Oliveira, Rachid Bencheikh, Patrícia Valentão, Paula B. Andrade, and Manuela Morato. 2022. "Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats" Molecules 27, no. 24: 8752. https://doi.org/10.3390/molecules27248752
APA StyleEl-Akhal, J., Oliveira, A. P., Bencheikh, R., Valentão, P., Andrade, P. B., & Morato, M. (2022). Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats. Molecules, 27(24), 8752. https://doi.org/10.3390/molecules27248752