Chemical Constituents of Macaranga occidentalis, Antimicrobial and Chemophenetic Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of Specialized Metabolites from M. occidentalis
2.2. Antimicrobial Activity of the Extract, Fractions, and Isolated Compounds
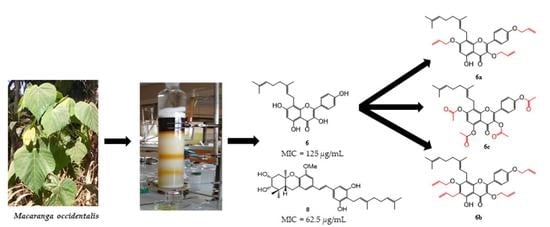
2.3. Alkylation and Acylation of Compound 6: Semisynthesis of Alkylated, and Acylated Derivatives 6a–c
2.3.1. Characterization of Compounds 6a–c
2.3.2. Antimicrobial Activity of Compounds 6a–c
2.4. Chemical Significance of the Isolated Compounds
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of the Isolated Compounds
3.5. Preparation of the Semisynthetic Derivatives
3.5.1. Allylation of Isomacarangin (6)
3.5.2. Acetylation of Isomacarangin (6)
3.6. Antimicrobial Assays
3.6.1. Antibacterial Activity
3.6.2. Antifungal Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yadav, S.; Kapley, A. Antibiotic resistance: Global health crisis and metagenomics. Biotechnol Rep. 2021, 29, e00604. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug. Resist. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouogo, N.D.C.; Tamokou, J.D.D.; Teponno, R.B.; Matsuete-Takongmo, G.; Voutquenne-Nazabadioko, L.; Tapondjou, L.A.; Ngnokam, D. Chemotaxonomy and antibacterial activity of the extracts and chemical constituents of Psychotria succulenta Hiern. (Rubiaceae). Biomed Res. Int. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Stafford, A.M. Plant cell cultures as a source of bioactive small molecules. Curr. Opin. Drug Discov. Dev. 2002, 5, 296–303. [Google Scholar]
- Magadula, J.J. Phytochemistry and pharmacology of the genus Macaranga: A review. J. Med. Plant. Res. 2014, 8, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Segun, P.A.; Ogbole, O.O.; Ismail, F.M.; Nahar, L.; Evans, A.R.; Ajaiyeoba, E.O.; Sarker, S.D. Bioassay-guided isolation and structure elucidation of cytotoxic stilbenes and flavonols from the leaves of Macaranga barteri. Fitoterapia 2019, 134, 151–157. [Google Scholar] [CrossRef]
- Schutz, B.A.; Wright, A.D.; Rali, T.; Sticher, O. Prenylated flavanones from the leaves of Macaranga pleiostemona. Phytochemistry 1995, 40, 1273–1277. [Google Scholar] [CrossRef]
- Péresse, T.; Jézéquel, G.; Allard, P.M.; Pham, V.C.; Huong, D.T.; Blanchard, F.; Bignon, J.; Lévaique, H.; Wolfender, J.-L.; Litaudon, M.; et al. Cytotoxic prenylated stilbenes isolated from Macaranga tanarius. J. Nat. Prod. 2017, 80, 2684–2691. [Google Scholar] [CrossRef]
- Kawakami, S.; Harinantenaina, L.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. Macaflavanones A-G, prenylated flavanones from the leaves of Macaranga tanarius. J. Nat. Prod. 2008, 71, 1872–1876. [Google Scholar] [CrossRef]
- Darmawan, A.; Kosela, S.; Kardono, L.B.; Syah, Y.M. Scopoletin, a coumarin derivative compound isolated from Macaranga gigantifolia. J. Appl. Pharm. Sci. 2012, 2, 175–177. [Google Scholar]
- Ngoumfo, R.M.; Ngounou, G.E.; Tchamadeu, C.V.; Qadir, M.I.; Mbazoa, C.D.; Begum, A.; Ngninzeko, F.N.; Lontsi, D.; Choudhary, M.I. Inhibitory effect of macabarterin, a polyoxygenated ellagitannin from Macaranga barteri, on human neutrophil respiratory burst activity. J. Nat. Prod. 2008, 71, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Tabopda, T.K.; Fotso, G.W.; Ngoupayo, J.; Mitaine-Offer, A.C.; Ngadjui, B.T.; Lacaille-Dubois, M.A. Antimicrobial dihydroisocoumarins from Crassocephalum biafrae. Planta. Med. 2009, 75, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Fotso, G.W.; Kamdem, L.M.; Dube, M.; Fobofou, S.A.; Ebene, A.N.; Arnold, N.; Ngadjui, B.T. Antimicrobial secondary metabolites from the stem barks and leaves of Monotes kerstingii Gilg (Dipterocarpaceae). Fitoterapia 2019, 137, 104239. [Google Scholar] [CrossRef] [PubMed]
- Minarti; Cahyana, A.H.; Darmawan, A. Secondary metabolite compound isolated from the leaves of Macaranga magna Turrill. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2175, p. 020029. [Google Scholar] [CrossRef]
- Bouzeko, I.L.T.; Dongmo, F.L.M.; Ndontsa, B.L.; Ngansop, C.A.N.; Keumoe, R.; Bitchagno, G.T.M.; Jouda, J.B.; Mbouangouere, R.; Tchegnitegni, B.T.; Boyom, F.F.; et al. Chemical constituents of Mussaenda erythrophylla Schumach. & Thonn. (Rubiaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 98, 104329. [Google Scholar] [CrossRef]
- Thang, P.T.; Dung, N.A.; Giap, T.H.; Oanh, V.T.K.; Hang, N.T.M.; Huong, T.T.; Thanh, L.N.; Huong, D.T.M.; Van, C.P. Preliminary study on the chemical constituents of the leaves of Macaranga balansae Gagnep. Vietnam J. Chem. 2018, 56, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Liu, X.Q.; Dong, J.X. Apigenin glycosides from Euphorbia humifusa wild. Acta Pharm. Sin. 2009, 44, 496–499. [Google Scholar]
- Huonga, D.T.M.; Anh, L.T.; Cuc, N.T.; Nhiem, N.X.; Tai, B.H.; Van Kiem, P.; Litaudon, M.; Dang, T.T.; Chau, M.V.; Pham, V.C. Cytotoxic prenylated flavonoids from the leaves of Macaranga indica. Phytochem. Lett. 2019, 34, 39–42. [Google Scholar] [CrossRef]
- Beutler, J.A.; Shoemaker, R.H.; Johnson, T.; Boyd, M.R. Cytotoxic geranyl stilbenes from Macaranga schweinfurthii. J. Nat. Prod. 1998, 61, 1509–1512. [Google Scholar] [CrossRef]
- Atta-Ur-Rahman; Ngounou, F.N.; Choudhary, M.I.; Malik, S.; Makhmoor, T.; Nur-E-Alam, M.; Zareen, S.; Lontsi, D.; Ayafor, J.F.; Sondengam, B.L. New antioxidant and antimicrobial ellagic acid derivatives from Pteleopsis hylodendron. Planta. Med. 2001, 67, 335–339. [Google Scholar] [CrossRef]
- Li, X.C.; Elsohly, H.N.; Hufford, C.D.; Clark, A.M. NMR assignments of ellagic acid derivatives. Magn. Reson. Chem. 1999, 37, 856–859. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Zhang, Z.L.; He, L.; Wang, Z.; Wang, G.S. Isolation and identification of the phenolic compounds from the roots of Sanguisorba officinalis L. and their antioxidant activities. Molecules 2012, 17, 13917–13922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pojchaijongdee, N.; Sotanaphun, U.; Limsirichaikul, S.; Poobrasert, O. Geraniinic acid derivative from the leaves of Phyllanthus reticulatus. Pharm. Biol. 2010, 48, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W. Antioxidant activity of brocchlin carboxylic acid and its methyl ester from Chrozophora brocchiana. Nat. Prod. Res. 2007, 21, 632–640. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Ishigoside, a new glyceroglycolipid isolated from the brown alga Ishige okamurae. Biotechnol. Bioprocess Eng. 2009, 14, 20–26. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta. Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widyawati, P.S.; Budianta, T.D.W.; Kusuma, F.A.; Wijaya, E.L. Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia less leaves extracts. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 850–855. [Google Scholar]
- Lim, T.Y.; Lim, Y.Y.; Yude, C.M. Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food chem. 2009, 114, 594–598. [Google Scholar] [CrossRef]
- Lim, T.Y.; Yude, C.M. Bioactivity of leaves of Macaranga species in tropical peat swamp and non-peat swamp environments. J. Trop. For. Sci. 2014, 26, 134–141. [Google Scholar]
- Salah, M.A.; Bedir, E.; Toyang, N.J.; Khan, I.A.; Harries, M.D.; Wedge, D.E. Antifungal clerodane from Macaranga monandra (L) Muell.et Arg. (Euphorbiaceae). J. Agric. Food Chem. 2003, 517, 7607–7610. [Google Scholar] [CrossRef]
- Osorio, M.; Carvajal, M.; Vergara, A.; Butassi, E.; Zacchino, S.; Mascayano, C.; Montoya, M.; Mejías, S.; Cortez-San, M.M.; Vásquez-Martínez, Y. Prenylated flavonoids with potential antimicrobial activity: Synthesis, biological activity, and in silico study. Int. J. Mol. Sci. 2021, 22, 5472. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.M.; Abdullahi, M.I.; Uba, A.; Umar, A. Prenylation of aromatic secondary metabolites: A new frontier for development of novel drugs. Trop. J. Pharm. Res. 2014, 13, 307–314. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, D.D. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 717–739. [Google Scholar] [CrossRef]
- Fotso, G.W.; Ngameni, B.; Storr, T.E.; Ngadjui, B.T.; Mafu, S.; Stephenson, G.R. Synthesis of novel stilbene–coumarin derivatives and antifungal screening of Monotes kerstingii-specialized metabolites against Fusarium oxysporum. Antibiotics 2020, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Iinuma, M.; Asai, F.; Ohyama, M.; Burandt, C.L. Flavonoids from the root and stem of Sophora tomentosa. Phytochemistry 1997, 46, 1431–1437. [Google Scholar] [CrossRef]
- Beutler, J.A.; McCall, K.L.; Boyd, M.R. A novel geranylflavone from Macaranga schweinfurthii. Nat.Prod. Lett. 1999, 13, 29–32. [Google Scholar] [CrossRef]
- Subramani, K.; Karnan, R.; Sajitha, M.; Anbarasan, P.M. New and rare acylated flavone glycosides from the aerial parts of Chrozophoro rottleri. J. Biochem. 2013, 7, 102–106. [Google Scholar]
- Zakaria, I.; Ahmat, N.; Jaafar, F.M.; Widyawaruyanti, A. Flavonoids with antiplasmodial and cytotoxic activities of Macaranga triloba. Fitoterapia 2012, 83, 968–972. [Google Scholar] [CrossRef]
- Matsunami, K.; Takamori, I.; Shinzato, T.; Aramoto, M.; Kondo, K.; Otsuka, H.; Takeda, Y. Radical-scavenging activities of new megastigmane glucosides from Macaranga tanarius (L) MÜLL.-ARG. Chem. Pharm. Bull. 2006, 54, 1403–1407. [Google Scholar] [CrossRef] [Green Version]
- Mouzié, C.M.; Guefack, M.G.F.; Kianfé, B.Y.; Serondo, H.U.; Ponou, B.K.; Siwe-Noundou, X.; Teponno, R.B.; Krause, R.W.M.; Kuete, V.; Tapondjou, L.A. A new chalcone and antimicrobial chemical constituents of Dracaena stedneuri. Pharmaceuticals 2022, 15, 725. [Google Scholar] [CrossRef]
- Dube, M.; Llanes, D.; Saoud, M.; Rennert, R.; Imming, P.; Haeberli, C.; Keiser, J.; Arnold, N. Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz. Natural fungal compounds and synthetic derivatives with in vitro anthelmintic activities and antiproliferative effects against two human cancer cell lines. Molecules 2022, 27, 2950. [Google Scholar] [CrossRef] [PubMed]
- Nkuété, A.H.L.; Kuete, V.; Gozzini, D.; Migliolo, L.; Oliveira, A.L.; Wabo, H.K.; Tane, P.; Vidari, G.; Efferth, T.; Franco, O.L. Anti-leukemia activity of semisynthetic phenolic derivatives from Polygonum limbatum Meisn. Chem. Cent. J. 2015, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]




| Compounds | KP NR 41897 | PA NR 48982 | SA NR 46003 | EC ATCC 25922 | SF NR 518 | CA NR-29340 | CK HM-1122 | CG |
|---|---|---|---|---|---|---|---|---|
| NR-51685 | ||||||||
| 3 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 4 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 5 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 6 | >500 | >500 | 125 | >500 | 500 | >500 | >500 | >500 |
| 7 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 8 | >500 | >500 | 250 | >500 | 250 | >500 | >500 | >500 |
| 9 | >500 | >500 | 125 | >500 | 500 | >500 | >500 | >500 |
| 10 | >500 | >500 | 62.5 | >500 | >500 | >500 | >500 | >500 |
| 11 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 12 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 13 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 15 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 16 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 17 | 250 | >500 | >500 | 500 | >500 | >500 | >500 | >500 |
| 6a | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 6b | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 6c | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| DCM/MeOH (1:1) | >1000 | 1000 | 500 | 1000 | 500 | >2000 | >2000 | >2000 |
| EtOAc | >1000 | 1000 | 500 | 1000 | 500 | >2000 | >2000 | |
| n-BuOH | >1000 | 1000 | 250 | 1000 | 1000 | 2000 | 2000 | |
| Ciprofloxacin | 0.015 | 0.031 | 0.015 | 0.031 | ||||
| Fluconazole | 0.0625 | 0.0765 | 0.153 |
| Position | 6a | 6b | 6c | |||
|---|---|---|---|---|---|---|
| δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | |
| 2 | 156.7 | 158.3 | 154.3 | |||
| 3 | 138.2 | 138.2 | 134.6 | |||
| 4 | 179.3 | 178.7 | 170.5 | |||
| 4a | 106.4 | 108.7 | 115.4 | |||
| 5 | 156.0 | 157.3 | 149.0 | |||
| 6 | 91.7 | 6.74, s | 112.3 | 111.7 | 8.31, s | |
| 7 | 163.0 | 162.1 | 155.2 | |||
| 8 | 113.0 | 112.3 | 126.4 | |||
| 8a | 158.6 | 153.2 | 155.6 | |||
| 1′ | 123.9 | 124.0 | 127.9 | |||
| 2′/6′ | 131.1 | 8.13 (d, 9.0) | 131.3 | 8.17 (d, 9.0) | 130.6 | 8.81 (d, 8.8) |
| 3′/5′ | 115.4 | 7.12 (d, 9.0) | 115.6 | 7.14 (d, 9.0) | 123.2 | 8.16 (d, 8.8) |
| 4′ | 161.6 | 161.8 | 154.2 | |||
| 1′′ | 22.0 | 3.38 (d, 7.2) | 23.1 | 3.41 (d, 6.8) | 23.8 | 4.13, brs |
| 2′′ | 122.9 | 5.26 (d, 1.5) | 123.5 | 5.28, m | 121.6 | 5.83, s |
| 3′′ | 135.5 | 135.8 | 136.9 | |||
| 4′′ | 40.2 | 1.96 (dd, 9.0; 6.3) | 39.6 | 1.93 (t, 7.3) | 40.3 | 2.76/2.84, m |
| 5′′ | 27.3 | 2.05, m | 27.2 | 2.05, s | 27.2 | 2.83, m |
| 6′′ | 125.1 | 5.05, m | 125.0 | 5.06, m | 124.9 | 5.86, m |
| 7′′ | 131.6 | 131.7 | 131.8 | |||
| 8′′ | 17.8 | 1.55, s | 17.4 | 1.54, s | 17.7 | 2.58, s |
| 9′′ | 25.7 | 1.60, s | 25.8 | 1.59, s | 25.7 | 2.42, s |
| 10′′ | 16.3 | 1.79, s | 16.4 | 1.77, s | 16.4 | 2.36, s |
| Allyl | ||||||
| 3-O-Allyl | 73.7 | 4.55 (d, 6.0) | 73.7 | 4.75 (d, 6.0) | ||
| 134.8 | 6.00, m | 134.8 | 5.90, m | |||
| 118.2 | 5.30, m | 118.4 | 5.16, m | |||
| 7-O-Allyl | 70.0 | 4.75, (d, 6.0) | 76.1 | 4.45, (d, 6.0) | ||
| 133.8 | 5.16, m | 134.7 | 5.90, m | |||
| 117.8 | 5.50, m | 117.4 | 5.26, m | |||
| 4′-O-Allyl | 69.5 | 4.69, (d, 6.0) | 69.5 | 4.70, (d, 6.0) | ||
| 134.2 | 6.13, m | 134.2 | 6.12, m | |||
| 117.9 | 5.52, m | 117.9 | 5.17, m | |||
| 6-C-Allyl | 28.5 | 3.54, m | ||||
| 137.3 | 6.05, m | |||||
| 115.7 | 7.14, m | |||||
| Acetyl | ||||||
| 3-Ac | 20.4 | 2.31, s | ||||
| 169.4 | ||||||
| 5-Ac | 21.1 | 2.32, s | ||||
| 168.3 | ||||||
| 7-Ac | 20.4 | 2.39, s | ||||
| 169.2 | ||||||
| 4′-Ac | 21.0 | 2.40, s | ||||
| 168.4 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamso, V.F.K.; Simo Fotso, C.C.; Kanko Mbekou, I.M.; Tousssie, B.T.; Ndjakou Lenta, B.; Boyom, F.F.; Sewald, N.; Frese, M.; Ngadjui, B.T.; Wabo Fotso, G. Chemical Constituents of Macaranga occidentalis, Antimicrobial and Chemophenetic Studies. Molecules 2022, 27, 8820. https://doi.org/10.3390/molecules27248820
Kamso VFK, Simo Fotso CC, Kanko Mbekou IM, Tousssie BT, Ndjakou Lenta B, Boyom FF, Sewald N, Frese M, Ngadjui BT, Wabo Fotso G. Chemical Constituents of Macaranga occidentalis, Antimicrobial and Chemophenetic Studies. Molecules. 2022; 27(24):8820. https://doi.org/10.3390/molecules27248820
Chicago/Turabian StyleKamso, Viviane Flore Kamlo, Christophe Colombe Simo Fotso, Ines Michèle Kanko Mbekou, Billy Tchegnitegni Tousssie, Bruno Ndjakou Lenta, Fabrice Fekam Boyom, Norbert Sewald, Marcel Frese, Bonaventure Tchaleu Ngadjui, and Ghislain Wabo Fotso. 2022. "Chemical Constituents of Macaranga occidentalis, Antimicrobial and Chemophenetic Studies" Molecules 27, no. 24: 8820. https://doi.org/10.3390/molecules27248820
APA StyleKamso, V. F. K., Simo Fotso, C. C., Kanko Mbekou, I. M., Tousssie, B. T., Ndjakou Lenta, B., Boyom, F. F., Sewald, N., Frese, M., Ngadjui, B. T., & Wabo Fotso, G. (2022). Chemical Constituents of Macaranga occidentalis, Antimicrobial and Chemophenetic Studies. Molecules, 27(24), 8820. https://doi.org/10.3390/molecules27248820