New Solid Forms of Nitrofurantoin and 4-Aminopyridine Salt: Influence of Salt Hydration Level on Crystal Packing and Physicochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure Analysis
2.2. Evaluation of the Formation Pathways of the NFT Salts in LAG
2.3. Thermal Analysis
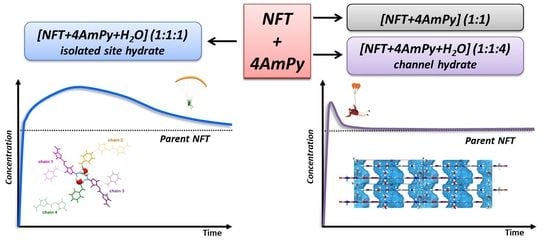
2.4. Powder Dissolution
3. Experimental Section
3.1. Materials
3.2. Sample Preparation
3.2.1. Solution Crystallization
3.2.2. Liquid-Assisted Grinding (LAG)
3.3. X-ray Diffraction
3.3.1. Single Crystal X-ray Diffraction (SCXRD)
3.3.2. Powder X-ray Diffraction (PXRD)
3.4. Thermal Analyses
3.4.1. Differential Scanning Calorimetry (DSC)
3.4.2. Thermogravimetric Analysis (TGA)
3.5. Dissolution Studies
3.6. High-Performance Liquid Chromatography (HPLC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aaltonen, J.; Allesø, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid form screening—A review. Eur. J. Pharm. Biopharm. 2009, 71, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Cabri, W.; Ghetti, P.; Alpegiani, M.; Pozzi, G.; Justo-Erbez, A.; Pérez-Martínez, J.I.; Villalón-Rubio, R.; Monedero-Perales, M.C.; Muñoz-Ruiz, A. Cefdinir: A comparative study of anhydrous vs. monohydrate form: Microstructure and tabletting behaviour. Eur. J. Pharm. Biopharm. 2006, 64, 212–221. [Google Scholar] [CrossRef]
- Liu, L.; An, Q.; Zhang, Y.; Sun, W.; Li, J.; Feng, Y.; Geng, Y.; Cheng, G. Improving the solubility, hygroscopicity and permeability of enrofloxacin by forming 1:2 pharmaceutical salt cocrystal with neutral and anionic co-existing p-nitrobenzoic acid. J. Drug Deliv. Sci. Technol. 2022, 76, 103732. [Google Scholar] [CrossRef]
- Malaj, L.; Censi, R.; Gashi, Z.; Di Martino, P. Compression behaviour of anhydrous and hydrate forms of sodium naproxen. Int. J. Pharm. 2010, 390, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Manin, A.N.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical salts of emoxypine with dicarboxylic acids. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, J.; Drozd, K.V.; Perlovich, G.L.; Balasubramanian, S.; Surov, A. Cocrystal and Coamorphous Solid Forms of Enzalutamide with Saccharin: Structural Characterization and Dissolution Studies. Cryst. Growth Des. 2022, 22, 6703–6716. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, H.; Wang, Y.; Xu, J.; Liu, Z.; Zhang, C. Recent advances in drug polymorphs: Aspects of pharmaceutical properties and selective crystallization. Int. J. Pharm. 2022, 611, 121320. [Google Scholar] [CrossRef]
- Drozd, K.V.; Manin, A.N.; Churakov, A.V.; Perlovich, G.L. Drug-drug cocrystals of antituberculous 4-aminosalicylic acid: Screening, crystal structures, thermochemical and solubility studies. Eur. J. Pharm. Sci. 2017, 99, 228–239. [Google Scholar] [CrossRef]
- Vasilev, N.A.; Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Perlovich, G.L. Novel cocrystals of itraconazole: Insights from phase diagrams, formation thermodynamics and solubility. Int. J. Pharm. 2021, 599, 120441. [Google Scholar] [CrossRef]
- Manin, A.N.; Surov, A.O.; Churakov, A.V.; Perlovich, G.L. Crystal Structures, Thermal Analysis, and Dissolution Behavior of New Solid Forms of the Antiviral Drug Arbidol with Dicarboxylic Acids. Crystals 2015, 5, 650–669. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Bookwala, M.; Thipsay, P.; Ross, S.; Zhang, F.; Bandari, S.; Repka, M.A. Preparation of a crystalline salt of indomethacin and tromethamine by hot melt extrusion technology. Eur. J. Pharm. Biopharm. 2018, 131, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Maddileti, D.; Swapna, B.; Nangia, A. High Solubility Crystalline Pharmaceutical Forms of Blonanserin. Cryst. Growth Des. 2014, 14, 2557–2570. [Google Scholar] [CrossRef]
- Drozd, K.V.; Manin, A.N.; Voronin, A.P.; Boycov, D.E.; Churakov, A.V.; Perlovich, G.L. A combined experimental and theoretical study of miconazole salts and cocrystals: Crystal structures, DFT computations, formation thermodynamics and solubility improvement. Phys. Chem. Chem. Phys. 2021, 23, 12456–12470. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, G.A.; Aburub, A.; Woods, T.A. Physical Stability of Salts of Weak Bases in the Solid-State. J. Pharm. Sci. 2011, 100, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, R.K.; Rout, S.R.; Kenguva, G.; Gorain, B.; Alhakamy, N.A.; Kesharwani, P.; Dandela, R. Recent advances in pharmaceutical cocrystals: From bench to market. Front. Pharmacol. 2021, 12, 780582. [Google Scholar] [CrossRef]
- Paulekuhn, G.S.; Dressman, J.B.; Saal, C. Trends in Active Pharmaceutical Ingredient Salt Selection based on Analysis of the Orange Book Database. J. Med. Chem. 2007, 50, 6665–6672. [Google Scholar] [CrossRef]
- Jurczak, E.; Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Pharmaceutical Hydrates Analysis—Overview of Methods and Recent Advances. Pharmaceutics 2020, 12, 959. [Google Scholar] [CrossRef]
- Griesser, U.J. The Importance of Solvates. In Polymorphism; Wiley: Hoboke, NJ, USA, 2006; pp. 211–233. [Google Scholar]
- Morris, K.R. Structural Aspects of Hydrates and Solvates. In Polymorphism in Pharmaceutical Solids; CRC Press: Boca Raton, FL, USA, 1999; pp. 125–182. [Google Scholar]
- Desiraju, G.R. Hydration in organic crystals: Prediction from molecular structure. J. Chem. Soc. Chem. Commun. 1991, 426–428. [Google Scholar] [CrossRef]
- Becker, A. Pharmaceutical salts of small molecule drugs: Opportunities and challenges. Eur. Pharm. Rev. 2014, 5. Available online: https://www.europeanpharmaceuticalreview.com/article/27753/pharmaceutical-salts-small-molecule-drugs/ (accessed on 28 October 2014).
- Manin, A.N.; Drozd, K.V.; Churakov, A.V.; Perlovich, G.L. Hydrogen Bond Donor/Acceptor Ratios of the Coformers: Do They Really Matter for the Prediction of Molecular Packing in Cocrystals? The Case of Benzamide Derivatives with Dicarboxylic Acids. Cryst. Growth Des. 2018, 18, 5254–5269. [Google Scholar] [CrossRef]
- Morris, K.; Rodriguez-Hornedo, N. Hydrates. In Encyclopaedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J., Eds.; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Vippagunta, S.R.; Brittain, H.G.; Grant, D.J.W. Crystalline solids. Adv. Drug Deliv. Rev. 2001, 48, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Sun, C.C. Superior Plasticity and Tabletability of Theophylline Monohydrate. Mol. Pharm. 2017, 14, 2047–2055. [Google Scholar] [CrossRef]
- Joiris, E.; Martino, P.D.; Malaj, L.; Censi, R.; Barthélémy, C.; Odou, P. Influence of crystal hydration on the mechanical properties of sodium naproxen. Eur. J. Pharm. Biopharm. 2008, 70, 345–356. [Google Scholar] [CrossRef]
- Manin, A.N.; Drozd, K.V.; Voronin, A.P.; Churakov, A.V.; Perlovich, G.L. A Combined Experimental and Theoretical Study of Nitrofuran Antibiotics: Crystal Structures, DFT Computations, Sublimation and Solution Thermodynamics. Molecules 2021, 26, 3444. [Google Scholar] [CrossRef]
- Voronin, A.P.; Vasilev, N.A.; Surov, A.O.; Churakov, A.V.; Perlovich, G.L. Exploring the solid form landscape of the antifungal drug isavuconazole: Crystal structure analysis, phase transformation behavior and dissolution performance. CrystEngComm 2021, 23, 8513–8526. [Google Scholar] [CrossRef]
- Surov, A.O.; Vasilev, N.A.; Vener, M.V.; Parashchuk, O.D.; Churakov, A.V.; Magdysyuk, O.V.; Perlovich, G.L. Pharmaceutical Salts of Fenbendazole with Organic Counterions: Structural Analysis and Solubility Performance. Cryst. Growth Des. 2021, 21, 4516–4530. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Stewardson, A.J.; Abbott, I.J.; Peleg, A.Y. Nitrofurantoin and fosfomycin for resistant urinary tract infections: Old drugs for emerging problems. Aust. Prescr. 2019, 42, 14–19. [Google Scholar] [CrossRef]
- Pienaar, E.W.; Caira, M.R.; Lötter, A.P. Polymorphs of nitrofurantoin. 2. Preparation and X-ray crystal structures of two anhydrous forms of nitrofurantoin. J. Crystallogr. Spectrosc. Res. 1993, 23, 785–790. [Google Scholar] [CrossRef]
- Pienaar, E.W.; Caira, M.R.; Lötter, A.P. Polymorphs of nitrofurantoin. I. Preparation and X-ray crystal structures of two monohydrated forms of nitrofurantoin. J. Crystallogr. Spectrosc. Res. 1993, 23, 739–744. [Google Scholar] [CrossRef]
- Otsuka, M.; Teraoka, R.; Matsuda, Y. Physicochemical Properties of Nitrofuratoin Anhydrate and Monohydrate and Their Dissolution. Chem. Pharm. Bull. 1991, 39, 2667–2670. [Google Scholar] [CrossRef] [Green Version]
- Alhalaweh, A.; George, S.; Basavoju, S.; Childs, S.L.; Rizvi, S.A.A.; Velaga, S.P. Pharmaceutical cocrystals of nitrofurantoin: Screening, characterization and crystal structure analysis. CrystEngComm 2012, 14, 5078–5088. [Google Scholar] [CrossRef]
- Segalina, A.; Pavan, B.; Ferretti, V.; Spizzo, F.; Botti, G.; Bianchi, A.; Pastore, M.; Dalpiaz, A. Cocrystals of Nitrofurantoin: How Coformers Can Modify Its Solubility and Permeability Across Intestinal Cell Monolayers. Cryst. Growth Des. 2022, 22, 3090–3106. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Volkova, T.V.; Vasilev, N.; Batov, D.; Churakov, A.V.; Perlovich, G.L. Extending the Range of Nitrofurantoin Solid Forms: Effect of Molecular and Crystal Structure on Formation Thermodynamics and Physicochemical Properties. Cryst. Growth Des. 2022, 22, 2569–2586. [Google Scholar] [CrossRef]
- Maity, D.K.; Paul, R.K.; Desiraju, G.R. Drug–Drug Binary Solids of Nitrofurantoin and Trimethoprim: Crystal Engineering and Pharmaceutical Properties. Mol. Pharm. 2020, 17, 4435–4442. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Babu, N.J.; Nangia, A. Nitrofurantoin–p-aminobenzoic acid cocrystal: Hydration stability and dissolution rate studies. J. Pharm. Sci. 2011, 100, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Vangala, V.R.; Chow, P.S.; Tan, R.B.H. Co-Crystals and Co-Crystal Hydrates of the Antibiotic Nitrofurantoin: Structural Studies and Physicochemical Properties. Cryst. Growth Des. 2012, 12, 5925–5938. [Google Scholar] [CrossRef]
- Sedehizadeh, S.; Keogh, M.; Maddison, P. The Use of Aminopyridines in Neurological Disorders. Clin. Neuropharmacol. 2012, 35, 191–200. [Google Scholar] [CrossRef]
- Strupp, M.; Feil, K.; Bardins, S.; Waidelich, R. 4-Aminopyridine Improves Lower Urinary Tract Symptoms in a Patient With Benign Prostatic Hyperplasia and Downbeat Nystagmus Syndrome. Int. Neurourol. J. 2014, 18, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Vangala, V.R.; Chow, P.S.; Tan, R.B.H. The solvates and salt of antibiotic agent, nitrofurantoin: Structural, thermochemical and desolvation studies. CrystEngComm 2013, 15, 878–889. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Roussel, P.; Perlovich, G.L. Diversity of crystal structures and physicochemical properties of ciprofloxacin and norfloxacin salts with fumaric acid. CrystEngComm 2018, 20, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiao, H.; Liu, N.; Zhang, B.; Shi, Q. Three new compounds derived from nitrofurantoin: X-ray structures and Hirshfeld surface analyses. Open J. Inorg. Chem. 2015, 5, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Vangala, V.R.; Chow, P.S.; Tan, R.B.H. Characterization, physicochemical and photo-stability of a co-crystal involving an antibiotic drug, nitrofurantoin, and 4-hydroxybenzoic acid. CrystEngComm 2011, 13, 759–762. [Google Scholar] [CrossRef]
- Caira, M.R.; Bettinetti, G.; Sorrenti, M. Structural relationships, thermal properties, and physicochemical characterization of anhydrous and solvated crystalline forms of tetroxoprim. J. Pharm. Sci. 2002, 91, 467–481. [Google Scholar] [CrossRef]
- Sheldrick, G. SADABS. Program for Scaling and Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997; p. 33. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]









| , °C | , % | , % | , J·g−1 | , kJ·mol−1 | |
|---|---|---|---|---|---|
| [NFT+4AmPy+H2O] (1:1:1) | 99.3 ± 0.2 | 5.14 | 5.09 | 131.6 ± 0.5 | 46.1 ± 0.5 |
| [NFT+4AmPy+H2O] (1:1:4) | 38.4 ± 0.2 | 17.83 | 17.76 | 324.3 ± 0.5 | 32.8 ± 0.5 |
| [NFT+4AmPy+H2O] (1:1:1) | [NFT+4AmPy+H2O] (1:1:4) | |
|---|---|---|
| Chemical formula | C8H5N4O5·C5H7N2·H2O | C8H5N4O5·C5H7N2·4(H2O) |
| Formula weight | 350.30 | 404.35 |
| Crystal system, space group | Monoclinic, P21/n | Orthorhombic, Pnma |
| Temperature, K | 150(2) | 100(2) |
| a, Å | 7.1941(3) | 18.6147(11) |
| b, Å | 19.5866(7) | 6.4828(4) |
| c, Å | 11.0933(4) | 14.9379(3) |
| β, ° | 98.2417(12) | 90 |
| Volume, Å3 | 1546.99(10) | 1802.64(19) |
| Z | 4 | 4 |
| Wavelength, Å | 0.71073 | 0.71073 |
| Radiation type | Mo Kα | Mo Kα |
| Dcalc, g·cm−3 | 1.504 | 1.490 |
| μ, mm−1 | 0.12 | 0.13 |
| Reflections collected | 20,175 | 33,823 |
| Independent reflections | 3368 | 2846 |
| Reflections with I > 2(I) | 2789 | 2649 |
| Rint | 0.036 | 0.049 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.036, 0.083, 1.05 | 0.043, 0.104, 1.13 |
| Parameters | 283 | 230 |
| Goodness-of-fit on F2 | 1.046 | 1.132 |
| Largest diff. peak/hole, e·Å−3 | 0.26, −0.17 | 0.46, −0.28 |
| CCDC number | 2216150 | 2216151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boycov, D.E.; Drozd, K.V.; Manin, A.N.; Churakov, A.V.; Perlovich, G.L. New Solid Forms of Nitrofurantoin and 4-Aminopyridine Salt: Influence of Salt Hydration Level on Crystal Packing and Physicochemical Properties. Molecules 2022, 27, 8990. https://doi.org/10.3390/molecules27248990
Boycov DE, Drozd KV, Manin AN, Churakov AV, Perlovich GL. New Solid Forms of Nitrofurantoin and 4-Aminopyridine Salt: Influence of Salt Hydration Level on Crystal Packing and Physicochemical Properties. Molecules. 2022; 27(24):8990. https://doi.org/10.3390/molecules27248990
Chicago/Turabian StyleBoycov, Denis E., Ksenia V. Drozd, Alex N. Manin, Andrei V. Churakov, and German L. Perlovich. 2022. "New Solid Forms of Nitrofurantoin and 4-Aminopyridine Salt: Influence of Salt Hydration Level on Crystal Packing and Physicochemical Properties" Molecules 27, no. 24: 8990. https://doi.org/10.3390/molecules27248990
APA StyleBoycov, D. E., Drozd, K. V., Manin, A. N., Churakov, A. V., & Perlovich, G. L. (2022). New Solid Forms of Nitrofurantoin and 4-Aminopyridine Salt: Influence of Salt Hydration Level on Crystal Packing and Physicochemical Properties. Molecules, 27(24), 8990. https://doi.org/10.3390/molecules27248990