Studies on the Selective Syntheses of Sodium Ditelluride and Dialkyl Ditellurides
Abstract
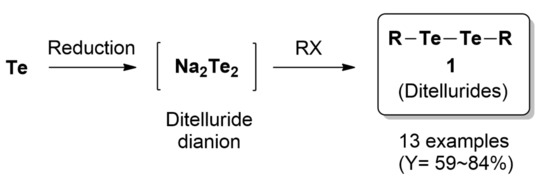
:1. Introduction
2. Results and Discussion
2.1. Optimization
2.2. Syntheses of Dialkyl Ditellurides 1
2.3. Studies on Reaction Pathways
3. Experimental
3.1. General Methods
3.2. General Procedure for the Syntheses of Dialkyl Ditellurides 1
3.2.1. 1,2-Di-n-hexyl Ditelluride (1a)
3.2.2. 1,2-Di-n-butyl Ditelluride (1b)
3.2.3. 1,2-Di-n-pentyl Ditelluride (1c)
3.2.4. 1,2-Di-n-heptyl Ditelluride (1d)
3.2.5. 1,2-Di-n-octyl Ditelluride (1e)
3.2.6. 1,2-Di-i-propyl Ditelluride (1f)
3.2.7. 1,2-Bis(3-pentyl) Ditelluride (1g)
3.2.8. 1,2-Bis(4-heptyl) Ditelluride (1h)
3.2.9. 1,2-Di-c-butyl Ditelluride (1i)
3.2.10. 1,2-Di-c-pentyl Ditelluride (1j)
3.2.11. 1,2-Di-c-hexyl Ditelluride (1k)
3.2.12. 1,2-Di-c-heptyl Ditelluride (1l)
3.2.13. 1,2-Bis(2-phenylethyl) Ditelluride (1m)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Steinbrenner, H.; Sies, H. Protection Against Reactive Oxygen Species by Selenoproteins. Biochim. Biophys. Acta 2009, 1790, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.S.; Hanna, M.S.; Cooperstein, D. Dietary Selenium and Selenoprotein Function. Med. Sci. Monit. 2012, 18, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belzile, N.; Chen, Y. Tellurium in the Environment: A Critical Review Focused on Natural Waters, Soils, Sediments and Airborne Particles. Appl. Geochem. 2015, 63, 83–92. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A Maverick among the Chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, J.T.; Coe, J.V.; McHugh, K.M.; Freidhoff, C.B.; Bowen, K.H. Photoelectron Spectroscopy of Selenium- and Tellurium-Containing Negative Ions: SeO2−, Se2−, and Te2−. J. Phys. Chem. 1989, 93, 1249–1254. [Google Scholar] [CrossRef]
- Ramadan, S.E.; Razak, A.A.; Ragab, A.M.; El-Meleigy, M. Incorporation of Tellurium into Amino Acids and Proteins in a Tellurium-Tolerant Fungi. Biol. Trace Elem. Res. 1989, 20, 225–232. [Google Scholar] [CrossRef]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-Like Activity of Selenides and Selenoxides: Experimental Evidence for the Involvement of Hydroxy Perhydroxy Selenane as the Active Species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef]
- Cunha, R.L.; Gouvea, I.E.; Juliano, L. A Glimpse on Biological Activities of Tellurium Compounds. An. Acad. Bras. Cienc. 2009, 81, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Lee, C.; Chang, H.; Chang, H. Antibacterial Activities of Tellurium Nanomaterials. Chem. Asian J. 2012, 7, 930–934. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium-Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef]
- Andersson, C.; Brattsand, R.; Hallberg, A.; Engman, L.; Persson, J.; Moldéus, P.; Cotgreave, I. Diaryl Tellurides as Inhibitors of Lipid Peroxidation in Biological and Chemical Systems. Free Radic. Res. 1994, 20, 401–410. [Google Scholar] [CrossRef]
- Engman, L.; Stern, D.; Pelcman, M.; Andersson, C.M. Thiol Peroxidase Activity of Diorganyl Tellurides. J. Org. Chem. 1994, 59, 1973–1979. [Google Scholar] [CrossRef]
- Sredni, B.; Caspi, R.R.; Klein, A.; Kalechmans, Y.; Danziger, Y.; BenYa’akov, M.; Tamari, T.; Shalit, F.; Albeck, M. A New Immunomodulating Compound (AS-101) with Potential Therapeutic Application. Nature 1987, 330, 173–176. [Google Scholar] [CrossRef]
- Giles, G.I.; Giles, N.M.; Collins, C.A.; Holt, K.; Fry, F.H.; Lowden, P.A.S.; Gutowski, N.J.; Jacob, C. Electrochemical, in Vitro and Cell Culture Analysis of Integrated Redox Catalysts: Implications for Cancer Therapy. Chem. Commun. 2003, 16, 2030–2031. [Google Scholar] [CrossRef]
- Lim, Y.J.; Shin, N.H.; Kim, C.; Kim, Y.E.; Cho, H.; Park, M.; Lee, S.H. An Efficient and Practical Method for the Selective Synthesis of Sodium Diselenide and Diorganyl Diselenides through Selenium Reduction. Tetrahedron 2020, 76, 131720. [Google Scholar] [CrossRef]
- Bhasin, K.K.; Gautam, A. A Novel and Convenient Synthesis for the Preparation of Dialkyl Tellurides and Ditellurides. Phosphorus Sulfur Relat. Elem. 1988, 38, 211–214. [Google Scholar] [CrossRef]
- Dabdoub, M.J.; Comasseto, J.V. Divinyl Ditellurides: Synthesis and Reactivity. J. Organomet. Chem. 1988, 344, 167–173. [Google Scholar] [CrossRef]
- Selvakumar, D.; Singh, R.; Nasim, M.; Mathjr, G.N. Synthesis of Bis(Alkyltelluro)Methanes and their Complexation with Cadmium(II). Phosphorus Sulfur Silicon Relat. Elem. 2001, 172, 247–259. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Li, Y.; Silverton, L.C.; Haasch, R.; Tong, Y.Y. Alkanetelluroxide-Protected Gold Nanoparticles. Langmuir 2008, 24, 7048–7053. [Google Scholar] [CrossRef]
- Singh, D.; Deobald, A.M.; Camargo, L.R.S.; Tabarelli, G.; Rodrigues, O.E.D.; Braga, A.L. An Efficient One-Pot Synthesis of Symmetrical Diselenides or Ditellurides from Halides with CuO Nanopowder/Se0 or Te0/Base. Org. Lett. 2010, 12, 3288–3291. [Google Scholar] [CrossRef] [PubMed]
- Engman, L.; Cava, M.P. Organotellurium Compounds 5. A Convenient Synthesis of some Aliphatic Ditellurides. Synth. Commun. 1982, 12, 163–165. [Google Scholar] [CrossRef]
- Webber, D.H.; Brutchey, R.L. Photolytic Preparation of Tellurium Nanorods. Chem. Commun. 2009, 38, 5701–5703. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.W.; Sharma, R.D. The Preparation of Di-t-Butyl Ditelluride and Di-t-Butyl Telluride and the 125Te NMR and Mössbauer Spectra of some Dialkyl Tellurides and Ditellurides. J. Organomet. Chem. 1983, 255, 61–70. [Google Scholar] [CrossRef]
- Shin, N.H.; Lim, Y.J.; Kim, C.; Kim, Y.E.; Jeong, Y.R.; Cho, H.; Park, M.; Lee, S.H. An Efficient Method for Selective Syntheses of Sodium Selenide and Dialkyl Selenides. Molecules 2022, 27, 5224. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Capperucci, A. Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium Under Lithium Triethylborohydride Conditions. Chemistry 2020, 2, 652–661. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyamae, T.; Nakai, I.; Kondoh, H.; Kawamoto, T.; Kobayashi, N.; Yasuda, S.; Yoshimura, D.; Ohta, T.; Nozoye, H.; et al. Adsorption States of Dialkyl Ditelluride Autooxidized Monolayers on Au(III). Langmuir 2005, 21, 3344–3353. [Google Scholar] [CrossRef]
- Botteselle, G.V.; Godoi, M.; Galetto, F.Z.; Bettanin, L.; Singh, D.; Rodrigues, O.E.D.; Braga, A.L. Microwave-Assisted One-Pot Synthesis of Symmetrical Diselenides, Ditellurides and Disulfides from Organoyl Iodides and Elemental Chalcogen Catalyzed by CuO Nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 186–193. [Google Scholar] [CrossRef]
- Li, J.Q.; Bao, W.L.; Lue, P.; Zhou, X. A Convenient Method for the Preparation of Dialkylditellurides and Dialkyldiselenides. Synth. Commun. 1991, 21, 799–806. [Google Scholar] [CrossRef]
- Duddeck, H.; Biallaβ, A. Substituent Effects and Stereochemistry in 125Te NMR Spectroscopy. Diorganyltellurium Dihalides and some Tellurides and Ditellurides. Magn. Reason. Chem. 1994, 32, 303–311. [Google Scholar] [CrossRef]
- Dabdoub, M.J.; Comasseto, J.V. Acetylenic Tellurides: Synthesis and Reactivity. Organometallics 1988, 7, 84–87. [Google Scholar] [CrossRef]





| Entry | Na2Te2 Formation a | Reaction with HexBr b | Yields (%) (1a:2a) c | Yields (%) (1a) c | ||
|---|---|---|---|---|---|---|
| NaBH4 (eq) | Temp (°C) | Time (h) | HexBr (eq) | |||
| 1 | 0.8 | 60 | 1 | 1.2 | 64 (11:1) | 59 |
| 2 | 80 | 0.5 | 68 (24:1) | 62 | ||
| 3 | 1.0 | 60 | 1 | 87 (14:1) | 76 | |
| 4 | 80 | 0.5 | 78 (13:1) | 69 | ||
| 5 | 1.2 | 60 | 1 | 90 (2.3:1) | 58 | |
| 6 | 60 | 2 | 93 (1.8:1) | 53 | ||
| 7 | 80 | 0.5 | 84 (2.3:1) | 54 | ||
| 8 | 80 | 1 | 91 (4.0:1) | 59 | ||
| 9 | 100 | 0.5 | 89 (2.0:1) | 54 | ||
| 10 | 100 | 1 | 91 (4.0:1) | 67 | ||
| 11 | 1.5 | 80 | 0.5 | 90 (0.76:1) | 30 | |

| Entry | Reaction with RX | Product | Yields (%) b | |
|---|---|---|---|---|
| RX | Time (h) | |||
| 1 | n-HexBr | 3 |  1a 1a | 76 |
| 2 | n-BuBr | 3 |  1b 1b | 81 |
| 3 | n-PenBr | 3 |  1c 1c | 80 |
| 4 | n-HepBr | 3 |  1d 1d | 76 |
| 5 | n-OctBr | 3 |  1e 1e | 84 |
| 6 | i-PrBr | 3 |  1f 1f | 79 |
| 7 | 3-Br-Pen | 3 |  1g 1g | 76 |
| 8 | 4-Br-Hep | 3 |  1h 1h | 76 |
| 9 | c-BuBr | 5 |  1i 1i | 67 |
| 10 | c-PenBr | 5 |  1j 1j | 66 |
| 11 | c-HexBr | 20 |  1k 1k | 59 |
| 12 | c-HepBr | 5 |  1l 1l | 79 |
| 13 |  | 5 |  1m 1m | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Lim, Y.J.; Kim, Y.E.; Cho, H.; Lee, S.H. Studies on the Selective Syntheses of Sodium Ditelluride and Dialkyl Ditellurides. Molecules 2022, 27, 8991. https://doi.org/10.3390/molecules27248991
Kim C, Lim YJ, Kim YE, Cho H, Lee SH. Studies on the Selective Syntheses of Sodium Ditelluride and Dialkyl Ditellurides. Molecules. 2022; 27(24):8991. https://doi.org/10.3390/molecules27248991
Chicago/Turabian StyleKim, Chorong, Yoo Jin Lim, Ye Eun Kim, Hyunsung Cho, and Sang Hyup Lee. 2022. "Studies on the Selective Syntheses of Sodium Ditelluride and Dialkyl Ditellurides" Molecules 27, no. 24: 8991. https://doi.org/10.3390/molecules27248991
APA StyleKim, C., Lim, Y. J., Kim, Y. E., Cho, H., & Lee, S. H. (2022). Studies on the Selective Syntheses of Sodium Ditelluride and Dialkyl Ditellurides. Molecules, 27(24), 8991. https://doi.org/10.3390/molecules27248991