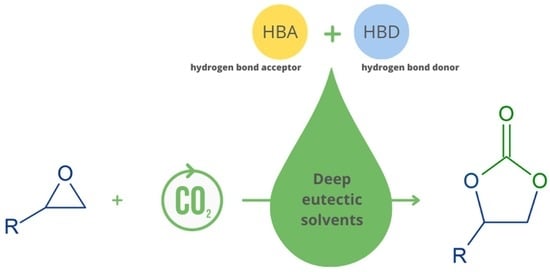
Deep Eutectic Solvents as Catalysts for Cyclic Carbonates Synthesis from CO2 and Epoxides
Abstract
:1. Introduction
2. Deep Eutectic Solvents as Catalysts for Cyclic Carbonates Synthesis from Epoxides and CO2
2.1. General Information
2.1.1. DES Synthesis
2.1.2. Mechanism of Cyclic Carbonate Synthesis from CO2 and Epoxides in the Presence of DES
2.1.3. The Influence of Water
2.1.4. The Influence of Hydroxyl Groups, pKa and Acidity of DES
2.1.5. The Influence of Temperature, Pressure and Reaction Time
2.1.6. The Influence of Substrates
2.1.7. Recycling of the DES-Based Catalysts
2.2. DES-Based Catalytic Systems Used in the Synthesis of Cyclic Carbonates—An Overview
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CAS SciFinder DataBase. Available online: https://scifinder-2n-1cas-1org-1q6ly29c00000.han.polsl.pl/search/reference/636e05ef0efde329e591e426/1 (accessed on 11 November 2022).
- Dalpozzo, R.; Ca, N.D.; Gabriele, B.; Mancuso, R. Recent advances in the chemical fixation of carbon dioxide: A green route to carbonylated heterocycle synthesis. Catalysts 2019, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Büttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent developments in the synthesis of cyclic carbonates from epoxides and CO2. Top Curr. Chem. 2017, 375, 50. [Google Scholar] [CrossRef] [PubMed]
- Nasirov, F.; Nasirli, E.; Ibrahimova, M. Cyclic carbonates synthesis by cycloaddition reaction of CO2 with epoxides in the presence of zinc-containing and ionic liquid catalysts. J. Iran. Chem. Soc. 2022, 19, 353–379. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Sum, Y.N.; Zhang, Y. Synthesis of cyclic carbonates with carbon dioxide and cesium carbonate. Green Chem. 2013, 15, 2086–2090. [Google Scholar] [CrossRef]
- Rollin, P.; Soares, L.K.; Barcellos, A.M.; Araujo, D.R.; Lenardão, E.J.; Jacob, R.G.; Perin, G. Five-membered cyclic carbonates: Versatility for applications in organic synthesis, pharmaceutical, and materials sciences. Appl. Sci. 2021, 11, 5024. [Google Scholar] [CrossRef]
- Wu, J.; Kozak, J.A.; Simeon, F.; Hatton, T.A.; Jamison, T.F. Mechanism-guided design of flow systems for multicomponent reactions: Conversion of CO2 and olefins to cyclic carbonates. Chem. Sci. 2014, 5, 1227–1231. [Google Scholar] [CrossRef]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Carbon dioxide fixation into organic compounds. In Carbon Dioxide Recovery and Utilization, 1st ed.; Aresta, M., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 211–260. [Google Scholar]
- Guo, L.; Lamb, K.J.; North, M. Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Caló, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef]
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kühn, F.E. Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. ChemSusChem 2015, 8, 2436–2454. [Google Scholar] [CrossRef]
- Siewniak, A.; Forajter., A.; Szymańska, K. Mesoporous silica-supported ionic liquids as catalysts for styrene carbonate synthesis from CO2. Catalysts 2020, 10, 1363. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, N.; Wei, W.; Sun, Y. Chemical fixation of carbon dioxide to propylene carbonate over amine-functionalized silica catalysts. Catal. Today 2006, 115, 102–106. [Google Scholar] [CrossRef]
- Shiels, R.A.; Jones, C.W. Homogeneous and Heterogeneous 4-(N,N-Dialkylamino) pyridines as effective single component catalysts in the synthesis of propylene carbonate. J. Mol. Catal. A Chem. 2007, 261, 160–166. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Sun, D.-S.; Zhou, H.; Zhang, W.-Z.; Lu, X.-B. CO2, COS and CS2 adducts of n-heterocyclic olefins and their application as organocatalysts for carbon dioxide fixation. Green Chem. 2015, 17, 4009. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A. Synthesis of organic carbonates. In Advances in Inorganic Chemistry, 1st ed.; Aresta, M., Eldik, R., Eds.; Academic Press Inc.: Waltham, MA, USA, 2014; Volume 66, pp. 25–81. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Liang, T.; Lin, X.; Shi, L.; Wang, F.; Sun, J. Cycloaddition of CO2 and epoxides catalyzed by dicationic ionic liquids mediated metal halide: Influence of the dication on catalytic activity. J. Mol. Catal. A Chem. 2015, 408, 242–249. [Google Scholar] [CrossRef]
- Peng, J.; Deng, Y. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar] [CrossRef]
- Jasiak, K.; Siewniak, A.; Kopczyńska, K.; Chrobok, A.; Baj., S. Hydrogensulphate ionic liquids as an efficient catalyst for the synthesis of cyclic carbonates from carbon dioxide and epoxides. J. Chem. Techol. Biotechnol. 2016, 91, 2827–2833. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.; Chen, P.; Liu, F.; Zhao, T. Effective synthesis of cyclic carbonates from CO2 and epoxides catalyzed by acetylcholine bromide-based deep eutectic solvents. J. CO2 Util. 2022, 58, 101936. [Google Scholar] [CrossRef]
- Yang, X.; Zou, Q.; Zhao, T.; Chen, P.; Liu, Z.; Liu, F.; Lin, Q. Deep eutectic solvents as efficient catalysts for fixation of CO2 to cyclic carbonates at ambient temperature and pressure through synergetic catalysis. ACS Sustain. Chem. Eng. 2021, 9, 10437–10443. [Google Scholar] [CrossRef]
- He, L.; Zhang, W.; Yang, Y.; Ma, J.; Liu, F.; Liu, M. Novel biomass-derived deep eutectic solvents promoted cycloaddition of CO2 with epoxides under mild and additive-free conditions. J. CO2 Util. 2021, 54, 101750. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg-Al mixed oxides as highly active acid-base catalysts for cycloaddition of carbon dioxide to epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar] [CrossRef]
- Tian, D.; Liu, B.; Gan, Q.; Li, H.; Darensbourg, D.J. Formation of cyclic carbonates from carbon dioxide and epoxides coupling reactions efficiently catalyzed by robust, recyclable one-component aluminum-salen complexes. ACS Catal. 2012, 2, 2029–2035. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Wu, K.; Su, T.; Hao, D.; Liao, W.; Zhao, Y.; Ren, W.; Deng, C.; Lü, H. Choline chloride-based deep eutectic solvents for efficient cycloaddition of CO2 with propylene oxide. Chem. Commun. 2018, 54, 9579–9582. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Row, K.H. Development of deep eutectic solvents for sustainable chemistry. J. Mol. Liq. 2022, 362, 119654. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A Review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; Hao, D.; Su, T.; Len, C.; Ren, W.; Lü, H. Synthesis cyclic carbonates with bmimcl-based ternary deep eutectic solvents system. J. CO2 Util. 2020, 40, 101250. [Google Scholar] [CrossRef]
- Lü, H.; Wu, K.; Zhao, Y.; Hao, L.; Liao, W.; Deng, C.; Ren, W. Synthesis of cyclic carbonates from CO2 and Propylene Oxide (PO) with Deep Eutectic Solvents (DESs) based on Amino Acids (AAs) and dicarboxylic acids. J. CO2 Util. 2017, 22, 400–406. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Vagnoni, M.; Samorì, C.; Galletti, P. Choline-based eutectic mixtures as catalysts for effective synthesis of cyclic carbonates from epoxides and CO2. J. CO2 Util. 2020, 42, 101302. [Google Scholar] [CrossRef]
- Claver, C.; Yeamin, M.B.; Reguero, M.; Masdeu-Bultó, A.M. Recent advances in the use of catalysts based on natural products for the conversion of CO2 into cyclic carbonates. Green Chem. 2020, 22, 7665–7706. [Google Scholar] [CrossRef]
- Ali, E.; Hadj-Kali, M.K.; Mulyono, S.; Alnashef, I. Analysis of operating conditions for CO2 capturing process using deep eutectic solvents. Int. J. Greenh. Gas Control 2016, 47, 342–350. [Google Scholar] [CrossRef]
- Dai, W.L.; Jin, B.; Luo, S.L.; Yin, S.F.; Luo, X.B.; Au, C.T. Cross-linked polymer grafted with functionalized ionic liquid as reusable and efficient catalyst for the cycloaddition of carbon dioxide to epoxides. J. CO2 Util. 2013, 3, 7–13. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Xin, H.; Zhao, P.; Gao, J.; Liu, M. Multifunctional phosphonium-based deep eutectic ionic liquids: Insights into simultaneous activation of CO2 and epoxide and their subsequent cycloaddition. ACS Sustain. Chem. Eng. 2019, 7, 16674–16681. [Google Scholar] [CrossRef]
- Sultana, K.; Rahman, M.T.; Habib, K.; Das, L. Recent advances in deep eutectic solvents as shale swelling inhibitors: A comprehensive review. ACS Omega 2022, 7, 28723–28755. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem. Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Zhao, P.; Xin, H.; Gao, J.; Liu, M. N-Hydroxysuccinimide based deep eutectic catalysts as a promising platform for conversion of CO2 into cyclic carbonates at ambient temperature. J. CO2 Util. 2019, 33, 419–426. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Yingcharoen, P.; Kongtes, C.; Arayachukiat, S.; Suvarnapunya, K.; Vummaleti, S.V.C.; Wannakao, S.; Cavallo, L.; Poater, A.; d’Elia, V. Assessing the PKa-dependent activity of hydroxyl hydrogen bond donors in the organocatalyzed cycloaddition of carbon dioxide to epoxides: Experimental and theoretical study. Adv. Synth. Catal. 2019, 361, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, X.; Lin, X.; Liang, L.; Gao, X.; Sun, J. Facile synthesis of [Urea-Zn]I2 eutectic-based ionic liquid for efficient conversion of carbon dioxide to cyclic carbonates. J. Mol. Catal. A Chem. 2016, 412, 20–26. [Google Scholar] [CrossRef]
- Adeleye, A.D.; Patel, D.; Niyogi, D.; Saha, B. Efficient and greener synthesis of propylene carbonate from carbon dioxide and propylene oxide. Ind. Eng. Chem. Res. 2014, 53, 18647–18657. [Google Scholar] [CrossRef]
- Yang, Z.Z.; He, L.N.; Miao, C.X.; Chanfreau, S. Lewis basic ionic liquids-catalyzed conversion of carbon dioxide to cyclic carbonates. Adv. Synth. Catal. 2010, 352, 2233–2240. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.; Zhou, Z.; Zhou, A. Imidazolium-based deep eutectic solvents as multifunctional catalysts for multisite synergistic activation of epoxides and ambient synthesis of cyclic carbonates. J. CO2 Util. 2021, 53, 101717. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Han, B.; Zhang, J.; Xie, Y.; Ma, X. Supported choline chloride/urea as a heterogeneous catalyst for chemical fixation of carbon dioxide to cyclic carbonates. Green Chem. 2007, 9, 169–172. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, H.; Lai, S.L.; Gao, J.; Gao, L. Lignin modified by deep eutectic solvents as green, reusable, and bio-based catalysts for efficient chemical fixation of CO2. React. Funct. Polym. 2020, 149, 104502. [Google Scholar] [CrossRef]
- Saptal, V.B.; Bhanage, B.M. Bifunctional ionic liquids derived from biorenewable sources as sustainable catalysts for fixation of carbon dioxide. ChemSusChem 2017, 10, 1145–1151. [Google Scholar] [CrossRef]
- García-Argüelles, S.; Ferrer, M.L.; Iglesias, M.; del Monte, F.; Gutiérrez, M.C. Study of superbase-based deep eutectic solvents as the catalyst in the chemical fixation of CO2 into cyclic carbonates under mild conditions. Materials 2017, 10, 759. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Morganstewart, H.; Tsang, S.C. Catalytic coupling of CO2 with epoxide over supported and unsupported amines. J. Phys. Chem. A 2009, 114, 3863–3872. [Google Scholar] [CrossRef]
- Sze, L.L.; Pandey, S.; Ravula, S.; Pandey, S.; Zhao, H.; Baker, G.A.; Baker, S.N. Ternary deep eutectic solvents tasked for carbon dioxide capture. ACS Sustain. Chem. Eng. 2014, 2, 2117–2123. [Google Scholar] [CrossRef]
- Dou, X.Y.; Wang, J.Q.; Du, Y.; Wang, E.; He, L.N. Guanidinium salt functionalized peg: An effective and recyclable homogeneous catalyst for the synthesis of cyclic carbonates from CO2 and epoxides under solvent-free conditions. Synlett 2007, 2007, 3058–3062. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.-Q.; Chen, J.-Y.; Cai, F.; Tian, J.-S.; Kong, D.-L.; He, L.-N. A Poly(Ethylene Glycol)-supported quaternary ammonium salt for highly efficient and environmentally friendly chemical fixation of CO2 with epoxides under supercritical conditions. Tetrahedron Lett. 2006, 47, 1271–1275. [Google Scholar] [CrossRef]
- Tak, R.K.; Patel, P.; Subramanian, S.; Kureshy, R.I.; Khan, N.U.H. Cycloaddition reaction of spiro-epoxy oxindole with CO2 at atmospheric pressure using deep eutectic solvent. ACS Sustain. Chem. Eng. 2018, 6, 11200–11205. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, F.; Xing, H.; Yang, Q.; Bao, Z.; Ren, Q. Efficient synthesis of cyclic carbonates from atmospheric CO2 using a positive charge delocalized ionic liquid catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2841–2846. [Google Scholar] [CrossRef]
- Xiao, L.; Su, D.; Yue, C.; Wu, W. Protic ionic liquids: A highly efficient catalyst for synthesis of cyclic carbonate from carbon dioxide and epoxides. J. CO2 Util. 2014, 6, 1–6. [Google Scholar] [CrossRef]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.C.M. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Guo, L.; Wang, Q.; Zhang, R.; Zhan, H.; Yi, L.; Chen, J.; Wu, X. Synthesis of TBAB-based deep eutectic solvents as the catalyst in the coupling reaction between CO2 and epoxides under ambient temperature. ChemistrySelect 2022, 7, e202202091. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.P.; Ji, X. Carbon dioxide capture with ionic liquids and deep eutectic solvents: A new generation of sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef]









| DES | HBA:HBD Molar Ratio | DES, mol% | Epoxide | Reaction Parameters | Yield, % | Selectivity, % | TON 1 | TOF 2, h−1 | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBA | HBD | Temperature, °C | Pressure, MPa | Time, h | ||||||||
| ChCl | urea | 1:2 | 1.0 | PO | 110 | - 3 - 4 | 10 4 | 99 99 5 | >99 | 95.0 99.0 | 9.5 24.8 | [50] |
| ZnI2 | urea | 1:3 | 1.2 | PO | 120 | 1.5 | 3 | 95 | 98 | 81.7 | 27.2 | [46] |
| TBAI | [Ch][His] | 1:1 | 20.0 | EP 6 | 70 | 0.1 1.0 | 30 2 | 92 7 98 7 | 99 99 | 4.6 4.9 | 0.2 2.5 | [52] |
| L-Pro + ZnBr2 | propanedioic acid | 1:2 | 2.0 | PO | 150 | 1.2 | 5 | 98 | - | 49.1 | 9.8 | [33] |
| TBD | benzyl alcohol | 1:1 | 1.0 | EP | 100 | 0.1 | 2 | 98 | 98 | 98.0 | 49.0 | [53] |
| ChCl | PEG200 | 1:2 | 2.1 | PO | 150 | 0.8 | 5 | 99 | >99 | 47.2 | 9.4 | [28] |
| ChCl | urea | 1:2 | 577.5 | n-benzyl spiro-epoxyoxindole | 70 | 0.1 | 2 | 98 | - | 0.2 | 0.1 | [58] |
| ChI | NHS | 1:2 | 6.0 | PO | 30 60 | 1.0 | 10 1 | 96 92 | 99 99 | 16.0 15.3 | 1.6 15.3 | [43] |
| TBPB | 3-AP | 1:2 | 4.5 | PO | 30 80 | 1.0 | 24 1 | 95 96 | >99 >99 | 21.1 21.3 | 0.9 21.3 | [40] |
| ChI | glycerol | 1:1 | 5.0 | SO | 80 | 0.1 | 7 | 90 | - | 18.0 | 2.6 | [36] |
| ChCl | PABA 8 | 1:1 | 10.0 | phenyl glycidyl ether | 110 | 1.0 | 3 | 99 | >99 | 9.9 | 3.3 | [51] |
| bmimCl | GA + BA 9 | 7:1:1 | 7.0 | PO | 70 | 0.8 | 7 | 98 | - | 14.0 | 2.0 | [32] |
| [DBUH][Br] | diethanoloamine | 2:1 | 20.0 | SO | r.t 10 60 | 0.1 | 48 5 | 97 >99 | >99 | 4.9 5.0 | 0.1 1.0 | [23] |
| emimI | m-DHB 11 | 2:1 | 10.0 | SO | r.t. | 0.1 | 24 | 99 | >99 | 9.9 | 0.4 | [49] |
| ChI | citric acid | 2:1 | 3.0 | PO | 70 | 0.5 | 3 | 98 | >99 | 32.7 | 10.9 | [24] |
| AcChBr | L-malic acid | 2:1 | 10.0 | SO | 80 | 0.1 | 2 | 98 | >99 | 9.8 | 4.9 | [22] |
| TBAB | citric acid | 2.5:1 | 0.9 | PO | 80 | 0.8 | 1 | 95 | 99 | 102.3 | 102.3 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mańka, D.; Siewniak, A. Deep Eutectic Solvents as Catalysts for Cyclic Carbonates Synthesis from CO2 and Epoxides. Molecules 2022, 27, 9006. https://doi.org/10.3390/molecules27249006
Mańka D, Siewniak A. Deep Eutectic Solvents as Catalysts for Cyclic Carbonates Synthesis from CO2 and Epoxides. Molecules. 2022; 27(24):9006. https://doi.org/10.3390/molecules27249006
Chicago/Turabian StyleMańka, Dorota, and Agnieszka Siewniak. 2022. "Deep Eutectic Solvents as Catalysts for Cyclic Carbonates Synthesis from CO2 and Epoxides" Molecules 27, no. 24: 9006. https://doi.org/10.3390/molecules27249006
APA StyleMańka, D., & Siewniak, A. (2022). Deep Eutectic Solvents as Catalysts for Cyclic Carbonates Synthesis from CO2 and Epoxides. Molecules, 27(24), 9006. https://doi.org/10.3390/molecules27249006