Nerve Regeneration Effect of a Composite Bioactive Carboxymethyl Chitosan-Based Nerve Conduit with a Radial Texture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Chitosan-Based Composite Bioactive NGC
2.2. The In Vitro Biocompatibility of CM-CTS
2.3. Protective Effect of CM-CTS on Hydrogen-Peroxide-Damaged Schwann RSC96 Cells
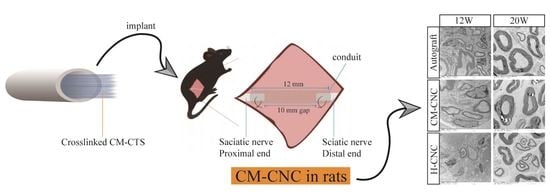
2.4. Functional Recovery of the Sciatic Nerve after a CM-CNC Transplant
2.5. Electrophysiological Recovery of a Damaged Nerve after a CM-CNC Transplant
2.6. Morphological and Ultrastructural Changes in Repaired Nerves by CM-CNC
2.7. Muscle Wet Weight and Motor Endplate Analysis after the CM-CNC Transplant
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cell Lines and Animals
3.3. Preparation of the Chitosan-Based Composite Bioactive Nerve Conduit
3.4. Biocompatibility of CM-CTS In Vitro
3.5. Bioactive Effects of CM-CTS In Vitro
3.6. Ten-Millimeter Sciatic Nerve Defect in SD Rats
3.7. Behavioral Analysis
3.8. Electrophysiological Measurements
3.9. Immunofluorescence Analysis
3.10. TEM Observation
3.11. Analysis of Target Muscles
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 2011, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ichihara, S.; Inada, Y.; Nakamura, T. Artificial nerve tubes and their application for repair of peripheral nerve injury: An update of current concepts. Injury 2008, 39 (Suppl. S4), 29–39. [Google Scholar] [CrossRef]
- Liao, I.C.; Wan, H.; Qi, S.; Cui, C.; Patel, P.; Sun, W.; Xu, H. Preclinical evaluations of acellular biological conduits for peripheral nerve regeneration. J. Tissue Eng. 2013, 4, 2041731413481036. [Google Scholar] [CrossRef] [Green Version]
- Aikeremujiang, M.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. Biomed. Res. Int 2015, 2015, 237507. [Google Scholar]
- Paprottka, F.J.; Wolf, P.; Harder, Y.; Kern, Y.; Paprottka, P.M.; Machens, H.G.; Lohmeyer, J.A. Sensory Recovery Outcome after Digital Nerve Repair in Relation to Different Reconstructive Techniques: Meta-Analysis and Systematic Review. Plast. Surg. Int. 2013, 2013, 704589. [Google Scholar] [CrossRef] [Green Version]
- Sva, B. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods—ScienceDirect. Acta Biomater. 2020, 106, 54–69. [Google Scholar]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Gan, L.; Zhao, L.; Zhao, Y.; Li, K.; Tong, Z.; Yi, L.; Wang, X.; Li, Y.; Tian, W.; He, X. Cellulose/soy protein composite-based nerve guidance conduits with designed microstructure for peripheral nerve regeneration. J. Neural Eng. 2016, 13, 056019. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ding, F.; Yang, Y.; Jie, L. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 2011, 93, 204–230. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Yang, Y.; Xing, X.; Yang, F.; Shan, P. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 78–85. [Google Scholar] [CrossRef]
- Lau, Y.T.; Kwok, L.F.; Tam, K.W.; Chan, Y.S.; Shum, K.Y.; Shea, K.H. Genipin-treated chitosan nanofibers as a novel scaffold for nerve guidance channel design. Colloids Surf. B Biointerfaces 2017, 162, 126–134. [Google Scholar] [CrossRef]
- Li, G.; Xue, C.; Wang, H.; Yang, X.; Zhao, Y.; Zhang, L.; Yang, Y. Spatially featured porous chitosan conduits with micropatterned inner wall and seamless sidewall for bridging peripheral nerve regeneration. Carbohydr. Polym. 2018, 194, S0144861718304351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Song, Y.; Qiao, J.; Yang, Y.; Zhang, W.; Liu, W.; Han, B. Rat sciatic nerve regeneration across a 10-mm defect bridged by a chitin/CM-chitosan artificial nerve graft. Int. J. Biol. Macromol. 2019, 129, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, J.; Ye, Z.; Xia, L.; Li, M.; Lv, B.; Shen, X.; Luo, Z. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng. Part A 2009, 15, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Berns, E.J.; Sur, S.; Pan, L.; Goldberger, J.E.; Suresh, S.; Zhang, S.; Kessler, J.A.; Stupp, S.I. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials 2014, 35, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- He, B.; Wu, F.; Fan, L.; Li, X.H.; Liu, Y.; Liu, Y.J.; Ding, W.J.; Deng, M.; Zhou, Y. Carboxymethylated chitosan protects Schwann cells against hydrogen peroxide-induced apoptosis by inhibiting oxidative stress and mitochondria dependent pathway. Eur. J. Pharmacol. 2018, 825, 48–56. [Google Scholar] [CrossRef]
- Wang, G.; Lu, G.; Ao, Q.; Gong, Y.; Zhang, X. Preparation of cross-linked carboxymethyl chitosan for repairing sciatic nerve injury in rats. Biotechnol. Lett. 2010, 32, 59–66. [Google Scholar] [CrossRef]
- Lu, G.; Kong, L.; Sheng, B.; Wang, G.; Gong, Y.; Zhang, X. Degradation of covalently cross-linked carboxymethyl chitosan and its potential application for peripheral nerve regeneration. Eur. Polym. J. 2007, 43, 3807–3818. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayan, R.; Maji, S.; Behera, S.; Kulanthaivel, S.; Maiti, T.K.; Banerjee, I.; Pal, K.; Giri, S. Gelatin/Carboxymethyl chitosan based scaffolds for dermal tissue engineering applications. Int. J. Biol. Macromol. 2016, 93, 1499–1506. [Google Scholar] [CrossRef]
- Zhu, S.; Ge, J.; Wang, Y.; Qi, F.; Ma, T.; Wang, M.; Yang, Y.; Liu, Z.; Huang, J.; Luo, Z. A synthetic oxygen carrier-olfactory ensheathing cell composition system for the promotion of sciatic nerve regeneration. Biomaterials 2014, 35, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Min, S.L.; Ju, H.L.; Si, H.P.; Yang, H.S. Micro-grooved nerve guidance conduits combined with microfiber for rat sciatic nerve regeneration. J. Ind. Eng. Chem. 2020, 90, 214–223. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; Mcinnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of Peripheral Nerves by Nerve Guidance Conduits: Influence of Design, Biopolymers, Cells, Growth Factors, and Physical Stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar]
- Xie, W.M.; Xu, P.X.; Wang, W.; Liu, Q. Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohyd. Polym. 2002, 50, 35–40. [Google Scholar] [CrossRef]
- Chang, G.; Dang, Q.; Liu, C.; Wang, X.; Song, H.; Gao, H.; Sun, H.; Zhang, B.; Cha, D. Carboxymethyl chitosan and carboxymethyl cellulose based self-healing hydrogel for accelerating diabetic wound healing. Carbohydr. Polym. 2022, 292, 119687. [Google Scholar] [CrossRef]
- Neubrech, F.; Sauerbier, M.; Moll, W.; Seegmüller, J.; Heider, S.; Harhaus, L.; Bickert, B.; Kneser, U.; Kremer, T. Enhancing the Outcome of Traumatic Sensory Nerve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair: A Randomized Controlled Bicentric Trial. Plast. Reconstr. Surg. 2018, 142, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcol, W.; Larysz-Brysz, M.; Kucharska, M.; Niekraszewicz, A.; Slusarczyk, W.; Kotulska, K.; Wlaszczuk, P.; Wlaszczuk, A.; Jedrzejowska-Szypulka, H.; Lewin-Kowalik, J. Reduction of Post-Traumatic Neuroma and Epineural Scar Formation in Rat Sciatic Nerve by Application of Microcrystallic Chitosan. Microsurgery 2011, 31, 642–649. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Park, C.J.; Park, S.A.; Yoon, T.G.; Lee, S.J.; Yum, K.W.; Kim, H.J. Bupivacaine Induces Apoptosis via ROS in the Schwann Cell Line. J. Dent. Res. 2005, 84, 852–857. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, Q. Curcumin accelerates the repair of sciatic nerve injury in rats through reducing Schwann cells apoptosis and promoting myelinization. Biomed. Pharmacother. 2017, 92, 1103–1110. [Google Scholar] [CrossRef]
- Purves, T.D.; Middlemas, A.; Agthong, S.; Jude, E.B.; Tomlinson, D.R. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. Faseb. J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 2508–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Tao, L.; Peng, L.; Mo, X.; Chen, H. Extracellular heat shock protein 72 protects schwann cells from hydrogen peroxide-induced apoptosis. J. Neurosci. Res. 2012, 90, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Tao, H.Y.; Liu, S.Q. Neuroprotective effects of carboxymethylated chitosan on hydrogen peroxide induced apoptosis in Schwann cells. Eur. J. Pharmacol. 2014, 740, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Shah, M.B.; Lee, P.; Yu, X. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. 2018, 73, 302–311. [Google Scholar] [CrossRef]
- Jiang, M.; Zhuge, X.; Yang, Y.; Gu, X.; Ding, F. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci. Lett. 2009, 454, 239–243. [Google Scholar] [CrossRef]
- Wang, X.; Wen, H.; Yong, C.; Jian, Y.; Jian, W.; Gu, X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 2005, 128, 1897. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, L.J.; Yannas, I.V.; Hsu, H.P.; Strichartz, G.; Spector, M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp. Neurol. 1998, 154, 315–329. [Google Scholar] [CrossRef]
- Marina, M.B.; Marie, J.P.; Birchall, M.A. Laryngeal reinnervation for bilateral vocal fold paralysis. Curr. Opin. Otolaryngol. 2011, 19, 434–438. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef]
- Jiang, Z.; Chi, J.; Li, H.; Wang, Y.; Liu, W.; Han, B. Effect of chitosan oligosaccharide-conjugated selenium on improving immune function and blocking gastric cancer growth. Eur. J. Pharm. 2021, 891, 173673. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater. Sci. Eng. C 2020, 109, 110564. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qiao, F.; Hou, W.; Yang, L.; Lv, Y. Graphene-based conductive fibrous scaffold boosts sciatic nerve regeneration and functional recovery upon electrical stimulation. Appl. Mater. Today 2020, 21, 100870. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, Z.; Wang, Y.; Xia, L.; Yu, S.; Li, H.; Zhang, W.; Liu, W.; Shao, K.; Han, B. Nerve Regeneration Effect of a Composite Bioactive Carboxymethyl Chitosan-Based Nerve Conduit with a Radial Texture. Molecules 2022, 27, 9039. https://doi.org/10.3390/molecules27249039
Zhang Y, Jiang Z, Wang Y, Xia L, Yu S, Li H, Zhang W, Liu W, Shao K, Han B. Nerve Regeneration Effect of a Composite Bioactive Carboxymethyl Chitosan-Based Nerve Conduit with a Radial Texture. Molecules. 2022; 27(24):9039. https://doi.org/10.3390/molecules27249039
Chicago/Turabian StyleZhang, Yijie, Zhiwen Jiang, Yanting Wang, Lixin Xia, Shuqin Yu, Hongjian Li, Wei Zhang, Wanshun Liu, Kai Shao, and Baoqin Han. 2022. "Nerve Regeneration Effect of a Composite Bioactive Carboxymethyl Chitosan-Based Nerve Conduit with a Radial Texture" Molecules 27, no. 24: 9039. https://doi.org/10.3390/molecules27249039
APA StyleZhang, Y., Jiang, Z., Wang, Y., Xia, L., Yu, S., Li, H., Zhang, W., Liu, W., Shao, K., & Han, B. (2022). Nerve Regeneration Effect of a Composite Bioactive Carboxymethyl Chitosan-Based Nerve Conduit with a Radial Texture. Molecules, 27(24), 9039. https://doi.org/10.3390/molecules27249039