Production and Structural Diversification of Withanolides by Aeroponic Cultivation of Plants of Solanaceae: Cytotoxic and Other Withanolides from Aeroponically Grown Physalis coztomatl
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Elucidation
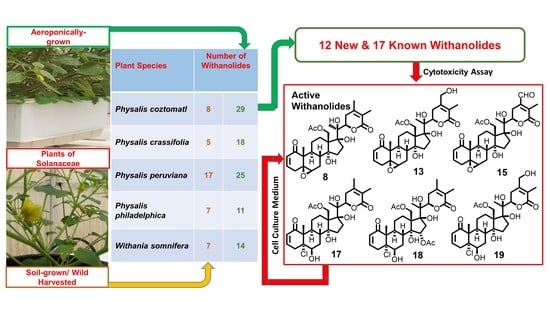
2.2. Biological Activities of Withanolides from P. coztomatl
3. Materials and Methods
3.1. General Methods and Materials
3.2. Aeroponic Cultivation and Harvesting of P. coztomatl
3.3. Extraction, Isolation and Identification of Withanolides
3.4. Acid Hydrolysis of Glycosides 9, 10, and 16
3.5. Cytotoxicity Assay
3.6. Conversion of 5α-Chloro-6β-hydroxy-5,6-dihydrophysachenolide D (17) to Physachenolide C (8)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Misico, R.I.; Nicotra, V.E.; Oberti, J.C.; Barboza, G.; Gil, R.R.; Burton, G. Withanolides and related steroids. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer: Wiena, Austria; New York, NY, USA, 2011; Volume 94, pp. 127–211. [Google Scholar]
- Cragg, G.M.; Schepartz, S.A.; Suffness, M.; Grever, M.R. The taxol supply crisis. New NCI policies for handling the large-scale production of novel natural product anticancer and anti-HIV agents. J. Nat. Prod. 1993, 56, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.J. Hydroponics Worldwide: State of the Art in Soilless Crop Production; International Center for Special Studies: Honolulu, HI, USA, 1985; p. 194. [Google Scholar]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Zobel, R.W. Steady-state control and investigation of root system morphology. In Applications of Continuous and Steady-State Methods to Root Biology; Torrey, J.G., Winship, L.J., Eds.; Kluwer: Dordrecht, The Netherlands, 1989; pp. 165–172. [Google Scholar]
- Weathers, P.J.; Zobel, R.W. Aeroponics for the culture of organisms, tissues and cells. Biotech. Adv. 1992, 10, 93–115. [Google Scholar] [CrossRef]
- Hayden, A.L. Aeroponic and hydroponic systems for medicinal herb, rhizome, and root crops. Hort. Sci. 2006, 41, 536–538. [Google Scholar] [CrossRef] [Green Version]
- NASA Spinoff. Progressive plant growing has business blooming. In Environmental and Agricultural Resources; NASA Spinoff: New York, NY, USA, 2006; pp. 64–77. [Google Scholar]
- Truong, B.; Beunard, P. Etude de la croissance racinaire de six variétés de riz pluvial en culture aéroponique. Premiers resultats. L’Agronomie Tropicale 1978, 33, 231–236. [Google Scholar]
- Wagner, R.E.; Wilkinson, H.T. An aeroponics system for investigating disease development on soybean taproots infected with Phytophthora sojae. Plant Dis. 1992, 76, 610–614. [Google Scholar] [CrossRef]
- Barak, P.; Smith, J.D.; Krueger, A.R.; Peterson, L.A. Measurement of short-term nutrient uptake rates in cranberry by aeroponics. Plant Cell Environ. 1996, 19, 236–242. [Google Scholar] [CrossRef]
- Buckseth, T.; Sharma, A.K.; Pande, K.K.; Singh, B.P.; Muthuraj, R. Methods of pre-basic seed potato production with special reference to aeroponic—A review. Sci. Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]
- Xu, Y.M.; Marron, M.T.; Seddon, E.; McLaughlin, S.P.; Ray, D.T.; Whitesell, L.; Gunatilaka, A.A.L. 2,3-Dihydrowithaferin A-3β-O-sulfate, a new potential prodrug of withaferin A from aeroponically grown Withania somnifera. Bioorg. Med. Chem. 2009, 17, 2210–2214. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L.; Xu, Y.; Wijeratne, E.M.K.; Whitesell, L.; Lindquist, S.L. Isolation and Preparation of Withaferin A Analogs for the Treatment of Proliferative, Neurodegenerative, Autoimmune and Inflammatory Diseases. International Patent No. WO 2010/030395 A3, 18 March 2010. [Google Scholar]
- Xu, Y.M.; Gao, S.; Bunting, D.P.; Gunatilaka, A.A.L. Unusual withanolides from aeroponically grown Withania somnifera. Phytochemistry 2011, 72, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Bunting, D.P.; Liu, M.X.; Bandaranayake, H.A.; Gunatilaka, A.A.L. 17β-Hydroxy-18-acetoxywithanolides from aeroponically grown Physalis crassifolia and their potent and selective cytotoxicity for prostate cancer cells. J. Nat. Prod. 2016, 79, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Liu, M.X.; Grunow, N.; Wijeratne, E.M.K.; Paine-Murrieta, G.; Felder, S.; Kris, R.M.; Gunatilaka, A.A.L. Discovery of potent 17β-hydroxywithanolides for castration-resistant prostate cancer by high-throughput screening of a natural products library for androgen-induced gene expression inhibitors. J. Med. Chem. 2015, 58, 6984–6993. [Google Scholar] [CrossRef]
- Xu, Y.M.; Wijeratne, E.M.K.; Babyak, A.L.; Marks, H.R.; Brooks, A.D.; Tewary, P.; Xuan, L.J.; Wang, W.Q.; Sayers, T.J.; Gunatilaka, A.A.L. Withanolides from aeroponically grown Physalis peruviana and their selective cytotoxicity to prostate cancer and renal carcinoma cells. J. Nat. Prod. 2017, 80, 1981–1991. [Google Scholar] [CrossRef]
- Xu, Y.M.; Wijeratne, E.M.K.; Brooks, A.D.; Tewary, P.; Xuan, L.J.; Wang, W.Q.; Sayers, T.J.; Gunatilaka, A.A.L. Cytotoxic and other withanolides from aeroponically grown Physalis philadelphica. Phytochemistry 2018, 152, 174–181. [Google Scholar] [CrossRef]
- Xu, G.B.; Xu, Y.M.; Wijeratne, E.M.K.; Ranjbar, F.; Liu, M.X.; Gunatilaka, A.A.L. Cytotoxic physalins from aeroponically grown Physalis acutifolia. J. Nat. Prod. 2021, 84, 187–194. [Google Scholar] [CrossRef]
- Wijeratne, E.M.; Xu, Y.M.; Scherz-Shouval, R.; Marron, M.T.; Rocha, D.D.; Liu, M.X.; Costa-Lotufo, L.V.; Santagata, S.; Lindquist, S.; Whitesell, L.; et al. Structure-activity relationships for withanolides as inducers of the cellular heat-shock response. J. Med. Chem. 2014, 57, 2851–2863. [Google Scholar] [CrossRef]
- Abraham, A.; Kirson, I.; Lavie, D.; Glotte, E. The withanolides of Withania somnifera chemotypes I and II. Phytochemistry 1975, 14, 189–194. [Google Scholar] [CrossRef]
- Lan, Y.; Chang, F.; Pan, M.; Wu, C.; Wu, S.; Chen, S.; Wang, S.; Wu, M.; Wu, Y. New cytotoxic withanolides from Physalis peruviana. Food Chem. 2009, 116, 462–469. [Google Scholar] [CrossRef]
- Maldonado, E.; Pérez-Castorena, A.L.; Garcés, C.; Martínez, M. Philadelphicalactones C and D and other cytotoxic compounds from Physalis philadelphica. Steroids 2011, 76, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, C.-M.; Gallagher, R.J.; Day, V.W.; Kindscher, K.; Timmermann, B.N. Withanolides from Physalis coztomatl. Phytochemistry 2015, 109, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Castorena, A.L.; Oropeza, R.F.; Vázquez, A.R.; Martínez, M.; Maldonado, E. Labdanes and withanolides from Physalis coztomatl. J. Nat. Prod. 2006, 69, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Brooks, A.D.; Wijeratne, E.M.K.; Henrich, C.J.; Tewary, P.; Sayers, T.J.; Gunatilaka, A.A.L. 17β-Hydroxywithanolides as sensitizers of renal carcinoma cells to tumor necrosis factor-α related apoptosis inducing ligand (TRAIL) mediated apoptosis: Structure–activity relationships. J. Med. Chem. 2017, 60, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Tewary, P.; Gunatilaka, A.A.L.; Sayers, T.J. Using natural products to promote caspase-8-dependent cancer cell death. Cancer Immunol. Immunother. 2017, 66, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Tewary, P.; Brooks, A.D.; Xu, Y.M.; Wijeratne, E.M.K.; Gunatilaka, A.A.L.; Sayers, T.J. A specific 17-beta-hydroxywithanolide (LG-02) sensitizes cancer cells to apoptosis in response to TRAIL and TLR3 ligands. American Association for Cancer Research Annual Meeting, Washington, DC, USA, 1–5 April 2007; AACR: Philadelphia, PA, USA. Cancer Res. 2017, 77 (Suppl. 13), 2159. [Google Scholar]
- Tewary, P.; Brooks, A.D.; Xu, Y.M.; Wijeratne, E.M.K.; Babyak, A.L.; Back, T.C.; Chari, R.; Evans, C.N.; Henrich, C.J.; Meyer, T.J.; et al. Small-molecule natural product physachenolide C potentiates immunotherapy efficacy by targeting BET proteins. Cancer Res. 2021, 81, 3374–3386. [Google Scholar] [CrossRef]
- Wijeratne, E.M.K.; Xu, Y.M.; Liu, M.X.; Inacio, M.C.; Brooks, A.D.; Tewary, P.; Sayers, T.J.; Gunatilaka, A.A.L. Ring A/B-modified 17β-hydroxywithanolide analogues as antiproliferative agents for prostate cancer and potentiators of immunotherapy for renal carcinoma and melanoma. J. Nat. Prod. 2021, 84, 3029–3038. [Google Scholar] [CrossRef]
- Adams, A.C.; Macy, A.M.; Kang, P.; Castro-Ochoa, K.F.; Wijeratne, E.M.K.; Xu, Y.M.; Liu, M.X.; Charos, A.; Bosenberg, M.W.; Gunatilaka, A.A.L.; et al. Physachenolide C induces complete regression of established murine melanoma tumors via apoptosis and cell cycle arrest. Trans. Oncol. 2022, 15, 101259. [Google Scholar] [CrossRef]
- Moiseeva, G.P.; Vasina, O.E.; Abubakirov, N.K. Withasteroids of Physalis. X. Circular dichroism of withasteroids from plants of the genus Physalis. Chem. Nat. Compd. 1990, 26, 308–312. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Shibata, K.; Umehara, K. Cell differentiation inducing steroids from Withania somnifera L. (DUN.). Chem. Pharm. Bull. 1999, 47, 1646–1649. [Google Scholar] [CrossRef] [Green Version]
- Kasal, A.; Budesinsky, M.; Griffiths, W.J. Spectroscopic methods of steroid analysis. In Steroid Analysis, 2nd ed.; Makin, H.L.J., Gower, D.B., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 27–161. [Google Scholar]
- Maldonado, E.; Torres, F.R.; Martínez, M.; Pérez-Castorena, A.L. 18-Acetoxywithanolides from Physalis chenopodifolia. Planta Med. 2004, 70, 59–64. [Google Scholar] [PubMed]
- Bessalle, R.; Lavie, D. Withanolide C, A chlorinated withanolide from Withania somnifera. Phytochemistry 1992, 31, 3648–3651. [Google Scholar] [CrossRef]
- Torres, F.R.; Pérez-Castorena, A.L.; Arredondo, L.; Toscano, R.A.; Nieto-Camacho, A.; Martínez, M.; Maldonado, E. Labdanes, withanolides, and other constituents from Physalis nicandroides. J. Nat. Prod. 2019, 82, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Gutiérrez, R.; Pérez-Castorena, A.L.; Martínez, M. Orizabolide, a new withanolide from Physalis orizabae. J. Mex. Chem. Soc. 2012, 56, 128–130. [Google Scholar] [CrossRef]
- Ozawa, M.; Morita, M.; Hirai, G.; Tamura, S.; Kawai, M.; Tsuchiya, A.; Oonuma, K.; Maruoka, K.; Sodeoka, M. Contribution of cage-shaped structure of physalins to their mode of action in inhibition of NF-κB activation. ACS Med. Chem. Lett. 2013, 4, 730–735. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.-T.; Liu, J.-K.; Li, B. Ten new withanolides from Physalis peruviana. Steroids 2012, 77, 36–44. [Google Scholar] [CrossRef]
- Nittala, S.S.; Vande, V.V.; Frolow, F.; Lavie, D. Chlorinated withanolides from Withania somnifera and Acnistus breviflorus. Phytochemistry 1981, 20, 2547–2552. [Google Scholar] [CrossRef]
- Pramanick, S.; Roy, A.; Ghosh, S.; Majumder, H.K.; Mukhopadhyay, S. Withanolide Z, a new chlorinated withanolide from Withania somnifera. Planta Med. 2008, 74, 1745–1748. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.I.; Hussain, S.; Yousuf, S.; Dar, A.; Mudassar; Rahman, A.-U. Chlorinated and diepoxy withanolides from Withania somnifera and their cytotoxic effects against human lung cancer cell line. Phytochemistry 2010, 71, 2205–2209. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, H.; Timmermann, B.N. Chlorinated withanolides from Withania somnifera. Phytochem. Lett. 2011, 4, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Chlorinated plant steroids and their biological activities. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 70–85. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, V.; Podesta, F.; Shamma, M.; Freyer, A. New withanolides from Jaborosa magellanica. J. Nat. Prod. 1991, 54, 554–563. [Google Scholar] [CrossRef]



| Plant | Cultivation Method/Source | Number of Withanolides Isolated a | Major Withanolides(% Yield) b | Refs and Notes |
|---|---|---|---|---|
| Withania somnifera | Aeroponics | 14 | Withaferin A (1) (0.42) | [15,17,23] |
| 2,3-Dihydrowithaferin A-3β-O-sulfate (2) (0.51) | ||||
| Wild-crafted (chemotype I) | 7 | Withaferin A (1) (0.23) | [24] | |
| Physalis crassifolia | Aeroponics | 18 | Physachenolide D (3) (0.30) | [18] |
| 15α-Acetoxyphysachenolide D (4) (0.03) | ||||
| Wild-crafted | 5 | Physachenolide D (3) (0.01) | [19] | |
| 15α-Acetoxyphysachenolide D (4) (0.005) | ||||
| Physalis peruviana | Aeroponics | 25 | Withanolide E (5) (0.18) | [20] |
| 4β-Hydroxywithanolide E (6) (0.15) | ||||
| Wild-crafted | 17 | Withanolide E (5) c | [25] | |
| 4β-Hydroxywithanolide E (6) (0.008) | ||||
| Physalis philadelphica | Aeroponics | 11 | Ixocarpalactone B (7) (0.10) | [21] |
| Wild-crafted | 7 | Ixocarpalactone B (7) (0.02) | [26] | |
| Physalis coztomatl | Aeroponics | 29 | Physachenolide C (8) (0.05) | This study |
| Physachenolide D (3) (0.02) | ||||
| Wild-crafted | 8 | Physachenolide C (8) d | [27] | |
| Physachenolide D (3) (0.02) |
| Position | 9 a | 10 a | 11 | 12 | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 204.4 | 204.7 | 204.6 | 203.9 | ||||
| 2 | 5.83 (br d, 9.6) | 127.8 | 5.84 (br d, 10.0) | 127.8 | 5.84 (dd, 10.0, 2.0) | 127.7 | 5.85 (dd, 10.0, 2.0) | 127.9 |
| 3 | 6.77 (br d, 9.6) | 145.7 | 6.77 (ddd, 10.0, 4.8, 2.8) | 145.4 | 6.78 (ddd, 10.0, 4.8, 2.4) | 145.9 | 6.76 (ddd, 10.0, 4.8, 2.4) | 145.3 |
| 4 | 3.28 (m) | 33.5 | 3.28 (m) | 33.5 | 3.27 (br d, 21.2) | 33.5 | 3.27 (br d, 21.6) | 33.4 |
| 2.83 (m) | 2.83 (dd, 21.6, 4.8) | 2.83 (dd, 21.2, 2.4) | 2.82 (dd, 21.6, 4.8) | |||||
| 5 | 135.2 | 136.1 | 135.0 | 135.3 | ||||
| 6 | 5.57 (br s) | 124.9 | 5.57 (d, 4.8) | 124.4 | 5.57 (br s) | 124.7 | 5.57 (d, 5.6) | 124.4 |
| 7 | 2.22 (m) | 25.5 | 2.00 (m) | 30.5 | 2.08 (m) | 25.3 | 2.10 (m) | 25.4 |
| 1.82 (m) | 1.62 (m) | 1.81 (m) | 1.81 (m) | |||||
| 8 | 1.85 (m) | 35.1 | 1.69 (m) | 31.9 | 1.82 (m) | 36.2 | 1.80 (m) | 36.4 |
| 9 | 2.37 (m) | 36.0 | 1.76 (m) | 43.4 | 2.07 (m) | 36.1 | 2.15 (m) | 36.1 |
| 10 | 50.8 | 50.6 | 50.6 | 50.8 | ||||
| 11 | 2.31 (m) | 22.0 | 2.34 (m) | 23.3 | 2.15 (m) | 22.3 | 2.21 (m) | 22.0 |
| 1.51 (m) | 1.52 (m) | 1.34 (m) | 1.34 (m) | |||||
| 12 | 2.34 (m) | 24.8 | 2.64 (m) | 25.3 | 2.09 (m) | 27.2 | 1.96 (m) | 27.1 |
| 1.95 (m) | 2.55 (m) | 1.81 (m) | 1.91 (m) | |||||
| 13 | 56.3 | 50.6 | 50.5 | 50.6 | ||||
| 14 | 83.4 | 1.76 (m) | 57.6 | 83.3 | 82.7 | |||
| 15 | 2.43 (m) | 41.2 | 2.09 (m) | 31.9 | 1.59 (dd, 12.2, 9.2) | 32.1 | 1.61 (m) | 32.4 |
| 2.17 (m) | 1.40 (m) | 1.39 (m) | ||||||
| 16 | 5.82 (br s) | 126.7 | 5.74 (br s) | 128.3 | 2.00 (m) | 21.2 | 1.86, (m) | 20.7 |
| 1.88, (m) | ||||||||
| 17 | 151.1 | 153.0 | 2.70 (t, 9.2) | 49.3 | 2.73 (t, 9.7) | 49.9 | ||
| 18 | 4.59 (d, 10.4) | 67.3 | 4.56 (d, 11.2) | 66.6 | 4.40 (d, 11.6) | 62.9 | 4.89, (d, 11.6) | 62.9 |
| 3.99 (d, 10.4) | 4.02 (d, 11.2) | 3.98 (d, 11.6) | 3.70, (d, 11.6) | |||||
| 19 | 1.22 (s) | 18.7 | 1.23 (s) | 18.9 | 1.20 (s) | 18.9 | 1.22 (s) | 18.8 |
| 20 | 74.4 | 74.7 | 76.1 | 76.5 | ||||
| 21 | 1.28 (s) | 24.8 | 1.30 (s) | 26.6 | 1.43 (s) | 23.4 | 1.40 (s) | 20.9 |
| 22 | 4.49 (m) | 80.8 | 4.45 (m) | 81.5 | 4.23 (d, 7.8) | 85.8 | 3.60 (d, 8.4) | 75.9 |
| 23 | 2.50–2.78 (m) | 25.1 | 2.38 (m) | 32.3 | 4.35 (d, 7.8) | 66.9 | 4.06 (dd, 8.4, 8.0) | 81.1 |
| 1.78 (m) | ||||||||
| 24 | 148.2 | 147.6 | 156.7 | 2.32 (m) | 51.0 | |||
| 25 | 123.4 | 123.5 | 124.5 | 2.29 (m) | 37.0 | |||
| 26 | 165.9 | 165.8 | 164.6 | 177.6 | ||||
| 27 | 1.85 (s) | 12.3 | 1.86 (s) | 12.3 | 4.34 (s) | 57.3 | 1.28 (d, 6.8) | 14.1 |
| 28 | 4.46 (m) | 67.7 | 4.45 (m) | 68.0 | 2.06 (s) | 15.4 | 3.83 (dd, 11.2, 2.0) | 63.3 |
| 3.64, (dd, 11.2, 7.6) | ||||||||
| OAc-18 | 2.07 (s) | 21.3 | 2.08 (s) | 21.3 | 2.07 (s) | 21.2 | 2.14 (s) | 21.2 |
| 171.4 | 171.8 | 170.7 | 169.7 | |||||
| Glc-1′ | 4.27 (d, 6.0) | 102.5 | 4.30 (d, 7.2) | 102.5 | ||||
| Glc-2′ | 3.38 (m) | 73.3 | 3.39 (m) | 73.4 | ||||
| Glc-3′ | 3.26 (m) | 75.8 | 3.28 (m) | 75.8 | ||||
| Glc-4′ | 3.55 (m) | 69.4 | 3.60 (m) | 69.8 | ||||
| Glc-5′ | 3.47 (m) | 76.4 | 3.51 (m) | 76.4 | ||||
| Glc-6′ | 3.81 (m) | 61.2 | 3.82 (brs) | 61.5 | ||||
| Position | 13 a | 14 a | 15 b | 16 c | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 205.4 | 205.4 | 203.1 | 205.0 | ||||
| 2 | 5.99 (dd, 10.0, 2.8) | 130.0 | 5.98 (dd, 10.0, 2.8) | 129.9 | 5.98 (dd, 10.0, 2.8) | 129.6 | 5.79 (dd, 10.0, 2.0) | 127.7 |
| 3 | 6.97 (ddd, 10.0, 6.0, 2.4) | 147.1 | 6.97 (ddd, 10.0, 6.0, 2.4) | 147.1 | 6.80 (ddd,10.0, 6.4, 2.0) | 144.1 | 6.74 (ddd, 10.0, 4.8, 2.4) | 145.9 |
| 4 | 2.96 (dt, 18.8, 2.4) | 33.8 | 2.96 (dt, 18.8, 2.4) | 33.8 | 2.93 (dt, 18.4, 2.4) | 32.8 | 3.23 (m) | 33.4 |
| 1.94 (m) | 1.94 (m) | 1.85 (m) | 2.79 (dd, 21.6, 4.8) | |||||
| 5 | 63.4 | 63.2 | 62.0 | 134.8 | ||||
| 6 | 3.23 (br s) | 65.0 | 3.20 (d, 2.0) | 65.0 | 3.15 (br s) | 63.9 | 5.57 (d, 5.6) | 125.3 |
| 7 | 1.95 (m) | 27.7 | 2.05 (m) | 27.4 | 1.94 (m) | 26.3 | 2.20 (m) | 25.3 |
| 1.87 (m) | 1.93 (m) | 1.79 (m) | ||||||
| 8 | 1.92 (m) | 35.5 | 2.65 (m) | 35.9 | 1.87 (m) | 34.2 | 1.87 (m) | 34.4 |
| 9 | 1.83 (m) | 38.7 | 1.89 (m) | 38.6 | 1.89 (m) | 36.8 | 2.28 (m) | 35.8 |
| 10 | 49.7 | 49.8 | 48.4 | 50.6 | ||||
| 11 | 2.03 (m) | 24.5 | 2.05 (m) | 24.5 | 2.06 (m) | 22.8 | 2.19 (m) | 22.2 |
| 1.38 (m) | 1.40 (m) | 1.34 (m) | 1.55 (m) | |||||
| 12 | 2.15 (m) | 26.6 | 2.24 (m) | 26.6 | 2.18 (m) | 25.5 | 2.20 (m) | 28.3 |
| 1.89 (m) | 1.85 (m) | 1.68 (m) | 1.47 (m) | |||||
| 13 | 58.6 | 58.6 | 57.5 | 52.2 | ||||
| 14 | 83.0 | 81.3 | 81.5 | 84.5 | ||||
| 15 | 1.62 (m) | 33.8 | 5.06 (dd, 9.2, 8.4) | 77.5 | 1.66 (m) | 32.9 | 2.29 (m) | 39.7 |
| 1.55 (m) | 1.59 (m) | 2.14 (m) | ||||||
| 16 | 2.56 (m) | 37.8 | 2.36 (m) | 43.8 | 2.64 (m) | 37.8 | 5.78 (br s) | 124.5 |
| 1.65 (m) | 2.24 (m) | 1.58 (m) | ||||||
| 17 | 88.9 | 85.8 | 87.9 | 155.9 | ||||
| 18 | 4.36 (d, 11.2) | 65.9 | 4.46 (d, 11.2) | 65.4 | 4.40 (d, 11.6) | 64.7 | 1.11 (s) | 22.3 |
| 4.30 (d, 11.2) | 4.32 (d, 11.2) | 4.28 (d, 11.6) | ||||||
| 19 | 1.19 (s) | 15.3 | 1.20 (s) | 15.4 | 1.20 (s) | 14.8 | 1.18 (s) | 18.6 |
| 20 | 79.8 | 80.0 | 79.2 | 74.6 | ||||
| 21 | 1.39 (s) | 19.0 | 1.35 (s) | 19.1 | 1.43 (s) | 19.0 | 1.26 (s) | 22.4 |
| 22 | 4.86 (dd, 13.6, 3.2) | 83.7 | 4.86 (dd, 13.6, 3.2) | 83.6 | 4.90 (dd, 13.6, 3.2) | 81.3 | 4.37 (t, 8.0) | 81.1 |
| 23 | 3.22 (dd, 18.8, 2.8) | 30.3 | 3.18 (dd, 20.4, 2.0) | 30.3 | 3.14 (m) | 24.5 | 2.63 (m) | 25.0 |
| 2.38 (m) | 2.37 (m) | 2.30, (m) | ||||||
| 24 | 154.9 | 154.9 | 142.3 | 149.0 | ||||
| 25 | 122.4 | 122.4 | 138.1 | 122.8 | ||||
| 26 | 169.1 | 169.0 | 165.6 | 166.2 | ||||
| 27 | 1.88 (s) | 12.1 | 1.87 (s) | 12.1 | 2.35 (br s) | 11.5 | 1.82 (s) | 11.9 |
| 28 | 4.36 (d, 13.6) | 61.9 | 4.37 (d, 14.0) | 61.8 | 10.30 (s) | 190.2 | 4.42 (br s) | 67.7 |
| 4.24 (d, 13.6) | 4.20 (d, 14.0) | |||||||
| OAc-18 | 2.13 (s) | 21.3 | 2.13 (s) | 21.3 | 2.15 (s) | 21.1 | ||
| 173.5 | 173.1 | 170.8 | ||||||
| OAc-15 | 2.06 (s) | 21.4 | ||||||
| 172.6 | ||||||||
| Glc-1′ | 4.23 (d, 8.0) | 102.5 | ||||||
| Glc-2′ | 3.26 (m) | 73.3 | ||||||
| Glc-3′ | 3.23 (m) | 75.9 | ||||||
| Glc-4′ | 3.39 (m) | 69.8 | ||||||
| Glc-5′ | 3.37 (m) | 76.4 | ||||||
| Glc-6′ | 3.79 (dd, 12.0, 2.8) | 61.5 | ||||||
| 3.71 (dd, 12.0, 4.4) | ||||||||
| Position | 17 | 18 | 19 a | 20 b | 21 b,c | ||||
|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | δC | |
| 1 | 201.3 | 201.0 | 204.1 | 204.8 | 205.3 | ||||
| 2 | 5.89 (dd, 10.0, 2.4) | 128.6 | 5.89 (dd, 10.0, 2.4) | 128.5 | 5.83 (dd, 10.0, 2.0) | 129.2 | 5.73 (dd, 10.0, 2.8) | 129.0 | 127.8 |
| 3 | 6.62 (br dd, 10.0, 3.6) | 141.2 | 6.63 (ddd, 10.0, 4.8, 2.4) | 141.5 | 6.75 (ddd, 10.0, 4.8, 2.0) | 144.1 | 6.48 (ddd, 10.0, 5.2, 2.0) | 139.6 | 142.2 |
| 4 | 3.49 (br d, 20.0) | 37.2 | 3.53 (dt, 20.4, 2.4) | 37.2 | 3.56 (br d, 20.0) | 38.7 | 2.95 (dt, 18.8, 2.4) | 27.4 | 35.0 |
| 2.49 (m) | 2.47 (dd, 20.4, 8.0) | 2.49 (dd, 20.0, 4.8) | 2.28 (m) | ||||||
| 5 | 79.5 | 79.0 | 82.3 | 81.3 | 77.3 | ||||
| 6 | 4.06 (br s) | 75.0 | 3.97 (br s) | 74.6 | 3.93 (t, 2.9) | 75.6 | 3.86 (brs) | 68.4 | 73.6 |
| 7 | 2.54 (m) | 29.7 | 2.61 (m) | 28.8 | 2.38 (m) | 30.4 | 1.98 (m) | 29.4 | 28.5 |
| 1.52 (m) | 1.54 (m) | 1.54 (m) | 1.37 (m) | ||||||
| 8 | 2.13 (m) | 34.6 | 2.82 (dt, 4.0, 12.0) | 34.8 | 2.26 (m) | 35.9 | 2.02 (m) | 33.9 | 33.4 |
| 9 | 2.76 (m) | 34.9 | 2.37 (dt, 4.0, 12.0) | 34.8 | 2.67 (m) | 36.4 | 2.61 (m) | 33.6 | 33.9 |
| 10 | 53.0 | 52.5 | 54.5 | 52.7 | 51.8 | ||||
| 11 | 2.50 (m) | 22.4 | 2.54 (m) | 22.5 | 2.39 (m) | 24.0 | 2.35 (m) | 22.6 | 22.6 |
| 1.22 (m) | 1.24 (m) | 1.24 (m) | 1.18 (m) | ||||||
| 12 | 2.40 (m) | 26.2 | 2.46 (m) | 26.1 | 2.28 (m) | 27.2 | 2.30 (m) | 26.1 | 26.1 |
| 1.81 (m) | 1.86 (m) | 1.94 (m) | 1.75 (m) | ||||||
| 13 | 57.8 | 58.0 | 58.9 | 57.5 | 57.4 | ||||
| 14 | 81.9 | 80.0 | 83.8 | 82.0 | 82.6 | ||||
| 15 | 1.68 (m) | 32.9 | 5.20 (t, 8.8) | 75.8 | 1.58–1.71 (m) | 33.6 | 1.63 (m) | 32.6 | 32.4 |
| 1.59 (m) | 1.51 (m) | ||||||||
| 16 | 2.71(m) | 37.9 | 2.53 (m) | 42.5 | 2.60 m | 37.8 | 2.61 (m) | 37.4 | 37.0 |
| 1.55 m | 2.26 (m) | 1.68 m | 1.47 (m) | ||||||
| 17 | 88.2 | 84.7 | 89.1 | 87.9 | 87.7 | ||||
| 18 | 4.43 (s) | 65.5 | 4.79 (d, 11.6) | 64.9 | 4.40 (s) | 66.4 | 4.37 (d, 11.2) | 65.5 | 65.3 |
| 4.22 (d, 11.6) | 4.30 (d, 11.2) | ||||||||
| 19 | 1.36 (s) | 16.1 | 1.36 (s) | 16.5 | 1.38 (s) | 17.0 | 1.22 s | 15.4 | 15.5 |
| 20 | 78.9 | 79.4 | 79.9 | 78.2 | 78.1 | ||||
| 21 | 1.41 (s) | 19.3 | 1.38 (s) | 19.2 | 1.40 (s) | 19.2 | 1.31 s | 18.4 | 18.1 |
| 22 | 4.90 (t, 8.4) | 79.7 | 4.91 (br d, 8.0) | 79.7 | 4.90 (m) | 84.0 | 4.84 (dd, 13.6, 3.2) | 80.8 | 81.0 |
| 23 | 2.53 (m) | 33.8 | 2.50 (m) | 33.8 | 2.40 (m) | 30.3 | 2.58 (m) | 33.7 | 33.6 |
| 2.44 (m) | |||||||||
| 24 | 149.7 | 149.9 | 154.9 | 150.6 | 151.0 | ||||
| 25 | 121.8 | 121.8 | 122.5 | 121.4 | 121.2 | ||||
| 26 | 165.7 | 165.7 | 169.2 | 167.2 | 167.5 | ||||
| 27 | 1.88 (s) | 12.4 | 1.88 (s) | 12.4 | 1.89 (s) | 12.1 | 1.82 s | 12.2 | 12.0 |
| 28 | 1.92 (s) | 20.7 | 1.92 (s) | 20.7 | 4.39 (d, 14.0) | 62.0 | 1.88 s | 20.6 | 20.5 |
| 4.23 (d, 14.0) | |||||||||
| OAc-18 | 2.07 (s) | 21.3 | 2.09 (s) | 21.4 | 2.12 (s) | 21.3 | 2.03 s | 21.3 | 21.2 |
| 170.4 | 171.4 | 173.6 | 171.1 | 171.2 | |||||
| OAc-15 | 2.08 (s) | 21.8 | |||||||
| 172.2 | |||||||||
| OMe | 2.93 s | 49.6 | |||||||
| Compound | Cell Line b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LNCaP | DU-145 | PC-3 | VCaP | ACHN | WI-38 | ||||||
| Activity | SI c | Activity | SI c | Activity | SI c | Activity | SI c | Activity | SI c | ||
| 8 | 0.03 ± 0.01 | 15.0 | 0.26 ± 0.01 | 1.7 | 0.06 ± 0.01 | 7.5 | 0.03 ± 0.01 | 15.0 | 1.02 ± 0.20 | 0.4 | 0.45 ± 0.11 |
| 13 | 2.78 ± 0.66 | >1.8 | >5.0 | 2.90 ± 0.32 | >1.7 | 1.11 ± 0.19 | >4.5 | >5.0 | >5.0 | ||
| 15 | 1.04 ± 0.18 | 3.6 | 2.67 ± 0.15 | 1.4 | 1.18 ± 0.21 | 3.2 | 0.82 ± 0.11 | 4.6 | 3.98 ± 0.10 | 0.9 | 3.77 ± 0.06 |
| 17 | 0.03 ± 0.01 | 17.0 | 0.67 ± 0.08 | 0.8 | 0.09 ± 0.01 | 5.7 | 0.08 ± 0.01 | 6.4 | 1.73 ± 0.18 | 0.3 | 0.51 ± 0.03 |
| 18 | 0.64 ± 0.16 | >7.8 | 4.53 ± 0.55 | >1.1 | 0.86 ± 0.19 | >5.8 | 0.27 ± 0.08 | >18.5 | >5.0 | >5.0 | |
| 19 | 1.98 ± 0.44 | >2.5 | >5.0 | 2.67 ± 0.21 | >1.9 | 1.26 ± 0.22 | 4.0 | >5.0 | >5.0 | ||
| Doxorubicin | 0.11 ± 0.02 | 0.04 ± 0.01 | 0.34 ± 0.05 | 0.67 ± 0.06 | 0.05 ± 0.01 | 0.80 ± 0.08 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.-M.; Wijeratne, E.M.K.; Liu, M.X.; Xuan, L.; Wang, W.; Gunatilaka, A.A.L. Production and Structural Diversification of Withanolides by Aeroponic Cultivation of Plants of Solanaceae: Cytotoxic and Other Withanolides from Aeroponically Grown Physalis coztomatl. Molecules 2022, 27, 909. https://doi.org/10.3390/molecules27030909
Xu Y-M, Wijeratne EMK, Liu MX, Xuan L, Wang W, Gunatilaka AAL. Production and Structural Diversification of Withanolides by Aeroponic Cultivation of Plants of Solanaceae: Cytotoxic and Other Withanolides from Aeroponically Grown Physalis coztomatl. Molecules. 2022; 27(3):909. https://doi.org/10.3390/molecules27030909
Chicago/Turabian StyleXu, Ya-Ming, E. M. Kithsiri Wijeratne, Manping X. Liu, Lijiang Xuan, Wenqiong Wang, and A. A. Leslie Gunatilaka. 2022. "Production and Structural Diversification of Withanolides by Aeroponic Cultivation of Plants of Solanaceae: Cytotoxic and Other Withanolides from Aeroponically Grown Physalis coztomatl" Molecules 27, no. 3: 909. https://doi.org/10.3390/molecules27030909
APA StyleXu, Y. -M., Wijeratne, E. M. K., Liu, M. X., Xuan, L., Wang, W., & Gunatilaka, A. A. L. (2022). Production and Structural Diversification of Withanolides by Aeroponic Cultivation of Plants of Solanaceae: Cytotoxic and Other Withanolides from Aeroponically Grown Physalis coztomatl. Molecules, 27(3), 909. https://doi.org/10.3390/molecules27030909