Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the HPTLC Conditions
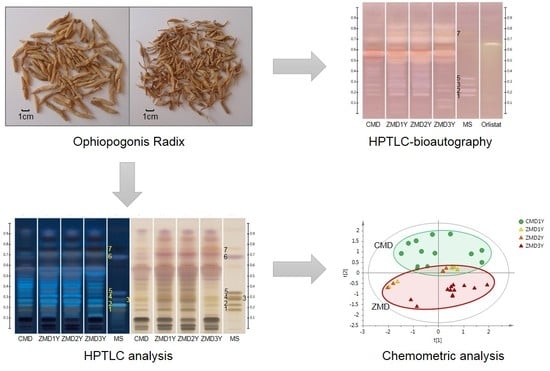
2.2. Comparison of Ophiopogonis Radix from Different Regions and/or Growth Years by Chemometric Approaches
2.3. Screening of Lipase Inhibitors from Ophiopogonis Radix by HPTLC-Bioautography
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Sample and Standard Solutions
3.3. HPTLC Analysis
3.4. Chemometric Analysis
3.5. HPTLC-Bioautographic Assay
3.6. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 December 2021).
- Chu, D.T.; Singh, V. Obesity and hypertension in Asia: Current status and challenges. Lancet. Reg. Health West. Pac. 2021, 15, 100243. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Cheung, B.M.Y.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 4, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.Y.; Jo, Y.H.; Kim, S.B.; Liu, Q.; Lee, J.W.; Mo, E.J.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions. Bioorg. Med. Chem. Lett. 2015, 25, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Tseng-Crank, J.; Corneliusen, B.; Yimam, M.; Hodges, M.; Hong, M.; Maurseth, C.; Oh, M.; Kim, H.; Chu, M.; et al. Lipase Inhibition and Antiobesity Effect of Atractylodes lancea. Planta Med. 2014, 80, 577–582. [Google Scholar] [CrossRef]
- Chen, T.G.; Li, Y.Y.; Zhang, L.W. Nine Different Chemical Species and Action Mechanisms of Pancreatic Lipase Ligands Screened Out from Forsythia suspensa Leaves All at One Time. Molecules 2017, 22, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wu, X.; Zhou, Y.; He, J. Camellia nitidissima Chi leaf as pancreatic lipase inhibitors: Inhibition potentials and mechanism. J. Food Biochem. 2021, 45, e13837. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Chen, X.J.; Wang, M.; Lin, L.G.; Wang, Y.T. Ophiopogon japonicus-A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 2016, 181, 193–213. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Wang, Y.; Feng, Y. MDG-1, an Ophiopogon polysaccharide, alleviates hyperlipidemia in mice based on metabolic profile of bile acids. Carbohydr. Polym. 2016, 150, 74–81. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Wang, X.; Feng, Y.; Wang, Y. MDG-1, an Ophiopogon polysaccharide, restrains process of non-alcoholic fatty liver disease via modulating the gut-liver axis. Int. J. Biol. Macromol. 2019, 141, 1013–1021. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.Y.; Yu, B.X. Methylophiopogonanone A, an Ophiopogon homoisoflavonoid, alleviates high-fat diet-induced hyperlipidemia: Assessment of its potential mechanism. Braz. J. Med. Biol. Res. 2020, 53, e9201. [Google Scholar] [CrossRef] [PubMed]
- Shimura, S.; Tsuzuki, W.; Suzuki, T. Determination of lipase activity in organic-solvent using fluorescent substrate and its application to the screening of lipase inhibitor. Anal. Sci. 1991, 7, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Q.; Zhang, D.; Yang, G.Y.; Zheng, Y.G.; Guo, L. Screening of Anti-Lipase Components of Artemisia argyi Leaves Based on Spectrum-Effect Relationships and HPLC-MS/MS. Front. Pharmacol. 2021, 12, 675396. [Google Scholar] [CrossRef] [PubMed]
- Banni, G.A.D.; Nasreddine, R.; Fayad, S.; Phu, C.N.; Rossi, J.C.; Leclercq, L.; Cottet, H.; Marchal, A.; Nehme, R. Screening for pancreatic lipase natural modulators by capillary electrophoresis hyphenated to spectrophotometric and conductometric dual detection. Analyst 2021, 146, 1386–1401. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, W.X.; Zhang, Y.Y.; Pan, X.; Duan, J.A. Rapid screening of lipase inhibitors in licorice extract by using porcine pancreatic lipase immobilized on Fe3O4 magnetic nanoparticles. Food Funct. 2021, 12, 5650–5657. [Google Scholar] [CrossRef]
- Ristivojevic, P.M.; Tahir, A.; Malfent, F.; Opsenica, D.M.; Rollinger, J.M. High-performance thin-layer chromatography/bioautography and liquid chromatography-mass spectrometry hyphenated with chemometrics for the quality assessment of Morus alba samples. J. Chromatogr. A 2019, 1594, 190–198. [Google Scholar] [CrossRef]
- Plazas, E.; Hagenow, S.; Avila Murillo, M.; Stark, H.; Cuca, L.E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ1-42 aggregation. Bioorg. Chem. 2020, 98, 103722. [Google Scholar] [CrossRef]
- Czernicka, L.; Ludwiczuk, A.; Roj, E.; Marzec, Z.; Jarzab, A.; Kukula-Koch, W. Acetylcholinesterase Inhibitors among Zingiber officinale Terpenes-Extraction Conditions and Thin Layer Chromatography-Based Bioautography Studies. Molecules 2020, 25, 1643. [Google Scholar] [CrossRef] [Green Version]
- Theiler, B.A.; Istvanits, S.; Zehl, M.; Marcourt, L.; Urban, E.; Caisa, L.O.E.; Glasl, S. HPTLC Bioautography Guided Isolation of α-Glucosidase Inhibiting Compounds from Justicia secunda Vahl (Acanthaceae). Phytochem. Anal. 2017, 28, 87–92. [Google Scholar] [CrossRef]
- Simões-Pires, C.A.; Hmicha, B.; Marston, A.; Hostettmann, K. A TLC bioautographic method for the detection of α- and β-glucosidase inhibitors in plant extracts. Phytochem. Anal. 2009, 20, 511–515. [Google Scholar] [CrossRef]
- Hassan, A.M.S. TLC Bioautographic Method for Detecting Lipase Inhibitors. Phytochem. Anal. 2012, 23, 405–407. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, J.; Tang, Q.; Wu, T.; Cheng, Z. A new TLC bioautographic assay for qualitative and quantitative estimation of lipase inhibitors. Phytochem. Anal. 2016, 27, 5–12. [Google Scholar] [CrossRef]
- Zhang, W.X.; Chao, I.C.; Hu, D.j.; Shakerian, F.; Ge, L.; Liang, X.; Wang, Y.; Zhao, J.; Li, S.P. Comparison of Antioxidant Activity and Main Active Compounds Among Different Parts of Alpinia officinarum Hance Using High-Performance Thin Layer Chromatography-Bioautography. J. AOAC Int. 2019, 102, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Ophiopogonis Radix. In Chinese Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1, pp. 162–163. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Dwarf lilyturf tuber. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; Supplement 10.1; pp. 4354–4355. [Google Scholar]
- Department of Health, Hong Kong Special Administrative Region, the People’s Republic of China. Radix Ophiopogonis. In Hong Kong Chinese Materis Medica Standards; Government of the Hong Kong Special Administrative Region, the People’s Republic of China: Hong Kong, China, 2010; Volume 3, pp. 165–174. [Google Scholar]
- Ge, Y.; Chen, X.; Gođevac, D.; Bueno, P.C.P.; Salomé Abarca, L.F.; Jang, Y.P.; Wang, M.; Choi, Y.H. Metabolic Profiling of Saponin-Rich Ophiopogon japonicus Roots Based on 1H NMR and HPTLC Platforms. Planta Med. 2019, 85, 917–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Chang, K.; Gao, P.; Qu, S.-b.; Zhang, Y.; Zhao, M.-b.; Shen, C.-k.; Le, Z.-y.; Jiang, Y.; Tu, P.-f.; et al. Analysis of Grading Evaluation Standard of Ophiopogonis Radix from Sichuan Province. Chin. J. Exp. Tradit. Med. Form. 2020, 26, 194–201. [Google Scholar] [CrossRef]
- Fichou, D.; Ristivojevic, P.; Morlock, G.E. Proof-of-Principle of rTLC, an Open-Source Software Developed for Image Evaluation and Multivariate Analysis of Planar Chromatograms. Anal. Chem. 2016, 88, 12494–12501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Xu, W.-f.; Shen, H.-y.; Shen, P.-q.; Zhang, J.; Wang, D.-d.; Xu, H.; Wang, H.; Yan, T.-t.; Wang, L.; et al. Comparison of bioactive components and pharmacological activities of Ophiopogon japonicas extracts from different geographical origins. J. Pharm. Biomed. Anal. 2017, 138, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.E.; Wang, Y.X.; Sun, P.; Liao, D.Q. Determination of saponin content in hang maidong and chuan maidong via HPLC-ELSD analysis. J. Anal. Methods Chem. 2016, 7214607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, C.-g.; Kang, C.-z.; Kang, L.-p.; Yang, J.; Wang, S.; He, Y.-l.; Deng, A.-p.; Wang, H.-y.; Huang, L.-q.; Guo, L.-p. Structural characterization and discrimination of Ophiopogon japonicas (Liliaceae) from different geographical origins based on metabolite profiling analysis. J. Pharm. Biomed. Anal. 2020, 185, 113212. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Compounds | Binding Energy (kcal/mol) | Hydrogen Bonds | Other Amino Acid Residues |
|---|---|---|---|
| Orlistat | −7.2 | HIS151, GLY76, PHE77 | THR255, ARG256, TRP252, HIS263, ALA259, LEU264, ASP79, HIS75, ILE78, ALA260, SER152, PHE215, LEU153, ALA178, TYR114, ILE209, PRO180 |
| Ophiopogonin D | −9.6 | PHE77 | ILE78, HIS263, PHE215, PRO180, TYR114, ILE209 |
| Ophiopojaponin C | −6.7 | ASP79 | ALA259, PHE215, LEU213, ILE209, VAL210 |
| Ophiopogonin D’ | −6.5 | - | HIS151, PHE215, ALA259, ILE78, LEU213, LEU264, ALA260 |
| Ophiopogonin C’ | −8.4 | HIS151, ASP79, ARG256 | VAL210, LEU213, TYR114, HIS263, PHE77, PHE215, TRP252 |
| Methylophiopogonanone B | −9.4 | SER152, HIS263 | ILE209, PRO180, TYR114, ALA259, ALA260, PHE215, LEU264, HIS151 |
| No. | Code | Regions | Growth Years |
|---|---|---|---|
| 1 | C1 | Laoma, Santai, Sichuan, China | 1 |
| 2 | C2 | Laoma, Santai, Sichuan, China | 1 |
| 3 | C3 | Laoma, Santai, Sichuan, China | 1 |
| 4 | C4 | Laoma, Santai, Sichuan, China | 1 |
| 5 | C5 | Laoma, Santai, Sichuan, China | 1 |
| 6 | C6 | Laoma, Santai, Sichuan, China | 1 |
| 7 | C7 | Xinde, Santai, Sichuan, China | 1 |
| 8 | C9 | Huanyuan, Santai, Sichuan, China | 1 |
| 9 | C10 | Laoma, Santai, Sichuan, China | 1 |
| 10 | C11 | Xinde, Santai, Sichuan, China | 1 |
| 11 | C12 | Laoma, Santai, Sichuan, China | 1 |
| 12 | C13 | Guangming, Santai, Sichuan, China | 1 |
| 13 | C14 | Lingxing, Santai, Sichuan, China | 1 |
| 14 | ZA1 | Muliwan, Sanmen, Zhejiang, China | 1 |
| 15 | ZA2 | Muliwan, Sanmen, Zhejiang, China | 2 |
| 16 | ZA3 | Muliwan, Sanmen, Zhejiang, China | 3 |
| 17 | ZB1 | Kanxiajin, Sanmen, Zhejiang, China | 1 |
| 18 | ZB2 | Kanxiajin, Sanmen, Zhejiang, China | 2 |
| 19 | ZB3 | Kanxiajin, Sanmen, Zhejiang, China | 3 |
| 20 | ZC1 | Baixi, Sanmen, Zhejiang, China | 1 |
| 21 | ZC2 | Baixi, Sanmen, Zhejiang, China | 2 |
| 22 | ZC3 | Baixi, Sanmen, Zhejiang, China | 3 |
| 23 | ZD1 | Nanlin, Sanmen, Zhejiang, China | 1 |
| 24 | ZD2 | Nanlin, Sanmen, Zhejiang, China | 2 |
| 25 | ZD3 | Nanlin, Sanmen, Zhejiang, China | 3 |
| 26 | ZE1 | Xiyang, Sanmen, Zhejiang, China | 1 |
| 27 | ZE2 | Xiyang, Sanmen, Zhejiang, China | 2 |
| 28 | ZE3 | Xiyang, Sanmen, Zhejiang, China | 3 |
| 29 | ZF1 | Fuhai, Cixi, Zhejiang, China | 3 |
| 30 | ZF2 | Fuhai, Cixi, Zhejiang, China | 3 |
| 31 | ZF3 | Fuhai, Cixi, Zhejiang, China | 3 |
| 32 | ZG1 | Shengshan, Cixi, Zhejiang, China | 3 |
| 33 | ZG2 | Shengshan, Cixi, Zhejiang, China | 3 |
| 34 | ZG3 | Shengshan, Cixi, Zhejiang, China | 3 |
| 35 | ZG4 | Shengshan, Cixi, Zhejiang, China | 3 |
| 36 | ZG5 | Shengshan, Cixi, Zhejiang, China | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, X.; Hong, H.-J.; Zhang, D.-Y.; Liu, Q.; Leong, F.; Yang, Q.; Hu, Y.-J.; Chen, X.-J. Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method. Molecules 2022, 27, 1155. https://doi.org/10.3390/molecules27041155
Hua X, Hong H-J, Zhang D-Y, Liu Q, Leong F, Yang Q, Hu Y-J, Chen X-J. Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method. Molecules. 2022; 27(4):1155. https://doi.org/10.3390/molecules27041155
Chicago/Turabian StyleHua, Xue, Hui-Jie Hong, Dai-Yan Zhang, Qiao Liu, Fong Leong, Qi Yang, Yuan-Jia Hu, and Xiao-Jia Chen. 2022. "Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method" Molecules 27, no. 4: 1155. https://doi.org/10.3390/molecules27041155
APA StyleHua, X., Hong, H. -J., Zhang, D. -Y., Liu, Q., Leong, F., Yang, Q., Hu, Y. -J., & Chen, X. -J. (2022). Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method. Molecules, 27(4), 1155. https://doi.org/10.3390/molecules27041155