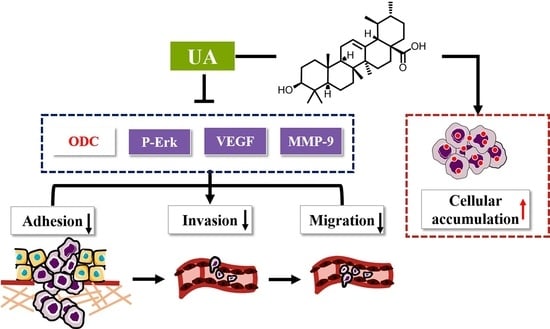
Inhibitory Effect of Ursolic Acid on the Migration and Invasion of Doxorubicin-Resistant Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. UA Reduced Adhesion of MCF-7/ADR Cells to HUVEC Cells
2.2. UA Inhibited Migration of MCF-7/ADR Cells
2.3. UA Reduced the Infiltration Ability of MCF-7/ADR Cells to HUVEC Cells
2.4. UA Had a Higher Binding Affinity for ODC Protein Compared with DFMO
2.5. UA Inhibited ODC, VEGF, MMP-9 Activity and Erk1/2 Phosphorylation in MCF-7/ADR Cells
2.6. UA Inhibited Polyamine Metabolism in MCF-7/ADR Cells
2.7. UA Increased the Intracellular Accumulation of Dox in MCF-7/ADR Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Adhesion Assay
4.4. Cell Migration Assay
4.5. Infiltration Assay of MCF-7/ADR Cells by Three-Dimensional MCT Infiltration Model
4.6. Molecular Docking
4.7. Western Blot Assay
4.8. Quantification of Metabolites Involved into Polyamine Metabolism
4.9. Intracellular Accumulation of Dox in MCF-7/ADR MCTs
4.10. UPLC-MS/MS Conditions
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.; Francavilla, C. ‘Omics Approaches to Explore the Breast Cancer Landscape. Front. Cell Dev. Biol. 2020, 7, 395. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Wang, Z.; Zhang, F.; Tian, R.; Ji, W.; Ren, X.; Niu, R. Rack1 Mediates the Interaction of P-Glycoprotein with Anxa2 and Regulates Migration and Invasion of Multidrug-Resistant Breast Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharm. 2018, 108, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.H.; Lane, D.J.R.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta 2018, 1862, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Weicht, R.; Schultz, C.; Geerts, D.; Uhl, K.; Bachmann, A. Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy. Med. Sci. 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.F.; Dong, C.D.; Wu, Q.; Jiang, Y.N.; Yao, K.; Zhang, J.; Zhao, S.M.; Ren, Y.; Yuan, Q.; Chen, X.H.; et al. Ornithine decarboxylase inhibition downregulates multiple pathways involved in the formation of precancerous lesions of esophageal squamous cell cancer. Mol. Carcinog. 2020, 59, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Arumugam, A.; Weng, Z.; Talwelkar, S.S.; Chaudhary, S.C.; Kopelovich, L.; Elmets, C.A.; Afaq, F.; Athar, M. Inhibiting Cycloxygenase and Ornithine Decarboxylase by Diclofenac and Alpha-Difluoromethylornithine Blocks Cutaneous SCCs by Targeting Akt-ERK Axis. PLoS ONE 2013, 8, e80076–e80087. [Google Scholar] [CrossRef]
- Hayes, C.S.; DeFeo, K.; Lan, L.; Paul, B.; Sell, C.; Gilmour, S.K. Elevated levels of ornithine decarboxylase cooperate with Raf/ERK activation to convert normal keratinocytes into invasive malignant cells. Oncogene 2006, 25, 1543–1553. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Fang, M.; Yu, H.; Liang, X.; Li, X.; Liu, X.; Chen, C.; Jia, J. Helicobacter pylori CagA induces ornithine decarboxylase upregulation via Src/MEK/ERK/c-Myc pathway: Implication for progression of gastric diseases. Exp. Biol. Med. 2012, 237, 435–441. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Sadremomtaz, A.; Mansouri, K.; Alemzadeh, G.; Safa, M.; Rastaghi, A.E.; Asghari, S.M. Dual blockade of VEGFR1 and VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway. Biochim. Biophys. Acta 2018, 1862, 2688–2700. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Hou, Y.; Li, A.; Sun, Y.; Li, S.; Zhao, Z. Yifei Tongluo, a Chinese Herbal Formula, Suppresses Tumor Growth and Metastasis and Exerts Immunomodulatory Effect in Lewis Lung Carcinoma Mice. Molecules 2019, 24, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C 2017, 71, 1231–1240. [Google Scholar] [CrossRef]
- Zong, L.; Cheng, G.; Liu, S.; Pi, Z.; Liu, Z.; Song, F. Reversal of multidrug resistance in breast cancer cells by a combination of ursolic acid with doxorubicin. J. Pharm. Biomed. Anal. 2019, 165, 268–275. [Google Scholar] [CrossRef]
- Zheng, G.; Shen, Z.; Xu, A.; Jiang, K.; Wu, P.; Yang, X.; Chen, X.; Shao, J. Synergistic Chemopreventive and Therapeutic Effects of Co-drug UA-Met: Implication in Tumor Metastasis. J. Agric. Food Chem. 2017, 65, 10973–10983. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, Y.; Shao, L.; Zhuang, J.L.; Huo, Q.; He, S.N.; Chen, S.Q.; Wang, J.; Xie, N. SIRT7 interacts with TEK (TIE2) to promote adriamycin induced metastasis in breast cancer. Cell. Oncol. 2021, 44, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; He, M.H.; Cui, L.Z.; Gao, M.H.; Zhang, M.; Yue, F.L.; Shi, T.F.; Yang, X.H.; Pan, Y.; Zheng, X.; et al. Chemotherapy exacerbates ovarian cancer cell migration and cancer stem cell-like characteristics through GLI1. Br. J. Cancer 2020, 122, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Pi, Z.; Liu, S.; Liu, Z.; Song, F. Metabolomics analysis of multidrug-resistant breast cancer cells in vitro using methyl-tert-butyl ether method. Rsc Adv. 2018, 8, 15831–15841. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Sivashanmugam, M.; K. N., S.; V., U. Virtual screening of natural inhibitors targeting ornithine decarboxylase with pharmacophore scaffolding of DFMO and validation by molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2019, 37, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.Y.; Zhan, J.Q.; Pan, J.; He, M.X.; Li, B.; Wang, J.; Ma, H.Y.; Wang, Y.L.; Liu, S. The rational discovery of multipurpose inhibitors of the ornithine decarboxylase. FASEB J. 2020, 34, 12907–12921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wang, D.M.; Loo, T.Y.; Cheng, Y.; Chen, L.L.; Shen, J.G.; Yang, D.P.; Chow, L.W.; Guan, X.Y.; Chen, J.P. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J. Ethnopharmacol. 2011, 133, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Daenen, L.G.M.; Roodhart, J.M.L.; van Amersfoort, M.; Dehnad, M.; Roessingh, W.; Ulfman, L.H.; Derksen, P.W.B.; Voest, E.E. Chemotherapy Enhances Metastasis Formation via VEGFR-1–Expressing Endothelial Cells. Cancer Res. 2011, 71, 6976–6985. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Gao, M.; Wang, Z.; Zhang, J.; Cui, W.; Li, J.; Zhu, X.; Zhang, H.; Yang, D.-H.; Xu, X. Curcumin reverses doxorubicin resistance in colon cancer cells at the metabolic level. J. Pharm. Biomed. Anal. 2021, 201, 114129–114138. [Google Scholar] [CrossRef] [PubMed]
- Kunjithapatham, R.; Karthikeyan, S.; Geschwind, J.F.; Kieserman, E.; Lin, M.; Fu, D.X.; Ganapathy-Kanniappan, S. Reversal of anchorage-independent multicellular spheroid into a monolayer mimics a metastatic model. Sci. Rep. 2014, 4, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.H.; Nicolson, G.L. Interactions of tumor cells with vascular endothelial cell monolayers: A model for metastatic invasion. Proc. Natl. Acad. Sci. USA 1979, 76, 5704–5708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayetano-Salazar, L.; Olea-Flores, M.; Zuniga-Eulogio, M.D.; Weinstein-Oppenheimer, C.; Fernandez-Tilapa, G.; Mendoza-Catalan, M.A.; Zacapala-Gomez, A.E.; Ortiz-Ortiz, J.; Ortuno-Pineda, C.; Navarro-Tito, N. Natural isoflavonoids in invasive cancer therapy: From bench to bedside. Phytother. Res. 2021, 35, 4092–4110. [Google Scholar] [CrossRef]
- van Iterson, V.; Leidenius, M.; von Smitten, K.; Bono, P.; Heikkila, P. VEGF-D in association with VEGFR-3 promotes nodal metastasis in human invasive lobular breast cancer. Am. J. Clin. Pathol. 2007, 128, 759–766. [Google Scholar] [CrossRef]
- Li, Q.Z.; Zuo, Z.W.; Zhou, Z.R.; Ji, Y. Polyamine homeostasis-based strategies for cancer: The role of combination regimens. Eur. J. Pharm. 2021, 910, 174456–174465. [Google Scholar] [CrossRef]
- Shukla-Dave, A.; Castillo-Martin, M.; Chen, M.; Lobo, J.; Gladoun, N.; Collazo-Lorduy, A.; Khan, F.M.; Ponomarev, V.; Yi, Z.; Zhang, W.; et al. Ornithine Decarboxylase Is Sufficient for Prostate Tumorigenesis via Androgen Receptor Signaling. Am. J. Pathol. 2016, 186, 3131–3145. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Stojanovic, I.; Stoimenov, T.J.; Pavlovic, D.; Kocic, G.; Bjelakovic, G.B.; Sokolovic, D.; Basic, J. Polyamines, folic acid supplementation and cancerogenesis. Pteridines 2017, 28, 115–131. [Google Scholar] [CrossRef]
- Geck, R.C.; Foley, J.R.; Stewart, T.M.; Asara, J.M.; Casero, R.A.; Toker, A. Inhibition of the polyamine synthesis enzyme ornithine decarboxylase sensitizes triple-negative breast cancer cells to cytotoxic chemotherapy. J. Biol. Chem. 2020, 295, 6263–6277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussai, F.; Egan, S.; Higginbotham-Jones, J.; Perry, T.; Beggs, A.; Odintsova, E.; Loke, J.; Pratt, G.; U., K.P.; Lo, A.; et al. Arginine dependence of acute myeloid leukemia blast proliferation: A novel therapeutic target. Blood 2015, 125, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Gao, Y.; Cao, Y.; Zhang, Y.; Xu, M.; Wang, Y.; Jing, Y.; Guo, S.; Jing, F.; Hu, X.; et al. Identification of arginine and its “Downstream” molecules as potential markers of breast cancer. IUBMB Life 2016, 68, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Miousse, I.R.; Tobacyk, J.; Quick, C.M.; Jamshidi-Parsian, A.; Skinner, C.M.; Kore, R.; Melnyk, S.B.; Kutanzi, K.R.; Xia, F.; Griffin, R.J.; et al. Modulation of dietary methionine intake elicits potent, yet distinct, anticancer effects on primary versus metastatic tumors. Carcinogenesis 2018, 39, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.F.; Wang, Y.B.; Wu, P. 5′-Methylthioadenosine and Cancer: Old molecules, new understanding. J. Cancer 2019, 10, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Avila, M.a.A.; García-Trevijano, E.R.; Lu, S.C.; Corrales, F.J.; Mato, J.M. Methylthioadenosine. Int. J. Biochem. Cell Biol. 2004, 36, 2125–2130. [Google Scholar] [CrossRef]
- Kirovski, G.; Stevens, A.P.; Czech, B.; Dettmer, K.; Weiss, T.S.; Wild, P.; Hartmann, A.; Bosserhoff, A.K.; Oefner, P.J.; Hellerbrand, C. Down-Regulation of Methylthioadenosine Phosphorylase (MTAP) Induces Progression of Hepatocellular Carcinoma via Accumulation of 5 ‘-Deoxy-5 ‘-Methylthioadenosine (MTA). Am. J. Pathol. 2011, 178, 1145–1152. [Google Scholar] [CrossRef]
- de Menezes, W.P.; Silva, V.A.O.; Gomes, I.N.F.; Rosa, M.N.; Spina, M.L.C.; Carloni, A.C.; Alves, A.L.V.; Melendez, M.; Almeida, G.C.; da Silva, L.S.; et al. Loss of 5 ‘-Methylthioadenosine Phosphorylase (MTAP) is Frequent in High-Grade Gliomas; Nevertheless, it is Not Associated with Higher Tumor Aggressiveness. Cells 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murin, R.; Vidomanova, E.; Kowtharapu, B.S.; Hatok, J.; Dobrota, D. Role of S-adenosylmethionine cycle in carcinogenesis. Gen. Physiol. Biophys. 2017, 36, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cameron, G.A.; Wallace, H.M. Decreased sensitivity to aspirin is associated with altered polyamine metabolism in human prostate cancer cells. Amino Acids 2016, 48, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Ghosh, S.; Joshi, M.B.; Ivanov, D.; Feder-Mengus, C.; Spagnoli, G.C.; Martin, I.; Erne, P.; Resink, T.J. Use of multicellular tumor spheroids to dissect endothelial cell–tumor cell interactions: A role for T-cadherin in tumor angiogenesis. FEBS Lett. 2007, 581, 4523–4528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, D.; Bauer, J.; Kromer, E.; Steinbach, P.; Riegger, G.; Hofstadter, F. Human follicular and papillary thyroid carcinoma cells interact differently with human venous endothelial cells. Thyroid 1995, 5, 155–164. [Google Scholar] [CrossRef]
- Stevens, A.P.; Dettmer, K.; Kirovski, G.; Samejima, K.; Hellerbrand, C.; Bosserhoff, A.K.; Oefner, P.J. Quantification of intermediates of the methionine and polyamine metabolism by liquid chromatography-tandem mass spectrometry in cultured tumor cells and liver biopsies. J. Chromatogr. A 2010, 1217, 3282–3288. [Google Scholar] [CrossRef] [PubMed]







| Cell Type | Fold Change 1 | ||
|---|---|---|---|
| +2 μM UA+ 10 μM Dox | +8 μM UA+ 10 μM Dox | +16 μM UA+ 10 μM Dox | |
| 2D cells | 1.15 * | 1.12 ** | 1.06 |
| 3D cells | 0.86 | 1.01 | 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, L.; Cheng, G.; Zhao, J.; Zhuang, X.; Zheng, Z.; Liu, Z.; Song, F. Inhibitory Effect of Ursolic Acid on the Migration and Invasion of Doxorubicin-Resistant Breast Cancer. Molecules 2022, 27, 1282. https://doi.org/10.3390/molecules27041282
Zong L, Cheng G, Zhao J, Zhuang X, Zheng Z, Liu Z, Song F. Inhibitory Effect of Ursolic Acid on the Migration and Invasion of Doxorubicin-Resistant Breast Cancer. Molecules. 2022; 27(4):1282. https://doi.org/10.3390/molecules27041282
Chicago/Turabian StyleZong, Li, Guorong Cheng, Jingwu Zhao, Xiaoyu Zhuang, Zhong Zheng, Zhiqiang Liu, and Fengrui Song. 2022. "Inhibitory Effect of Ursolic Acid on the Migration and Invasion of Doxorubicin-Resistant Breast Cancer" Molecules 27, no. 4: 1282. https://doi.org/10.3390/molecules27041282
APA StyleZong, L., Cheng, G., Zhao, J., Zhuang, X., Zheng, Z., Liu, Z., & Song, F. (2022). Inhibitory Effect of Ursolic Acid on the Migration and Invasion of Doxorubicin-Resistant Breast Cancer. Molecules, 27(4), 1282. https://doi.org/10.3390/molecules27041282