Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits
Abstract
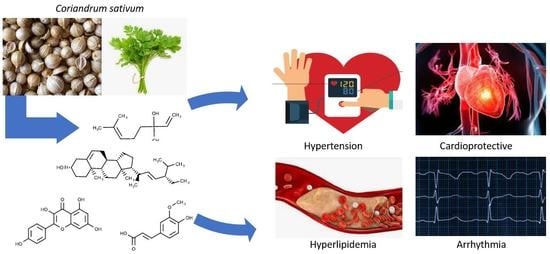
:1. Introduction
1.1. Botanical Description and Taxonomy
1.2. Ethnomedicinal Uses
1.3. Phytochemistry
| Phytochemical Components | Chemical Class | Plant Part | Extraction Solvent/Method | Reference |
|---|---|---|---|---|
| Ferulic acid, Gallic acid, and Caffeic acid | Phenolic acids | Above-ground parts | Ether, ethyl acetate, butanol, and 2-ethyl acetate extracts | [47] |
| Salicylic acid | Benzoic acid derivative | |||
| Esculetin, Esculin, Scopoletin 4-Hydroxycoumarin, Umbelliferone, and Dicoumarin | Coumarins | |||
| Hyperoside, rutin, hesperidin, vicenin, diosmin, luteolin, apigenin, orientine, dihydroquercetin, catechin, and arbutin | Flavonoids | |||
| β-carotene and total carotenoids | Carotenoids | Leaves at mature and young plant stage, fresh and dry seeds | Ice-cold acetone was then partitioned against petroleum ether. | [69] |
| α-, β-, γ- δ- tocopherols, and α-, γ-tocotrienols | Tocols | Whole fruit, pericarp, and seeds | Extracted with n-hexane | [70] |
| Petroselinic acid, linoleic acid, palmitic acid, and oleic acid | Fatty acids | Boiled in water, then grounded using a mixture of chloroform/methanol/hexane and finally separated by thin-layer chromatography | ||
| Stigmasterol, β-sitosterol, δ-stigmasterol | Sterols | Seed and pericarp of coriander fruit | Extracted with n-hexane in a Soxhlet apparatus | [71] |
| Linalool, camphor, and geraniol | Essential oils | Hydrodistillation followed by extraction with 2-methylbutane |
2. Results
3. Discussion
3.1. Hypolipidemic Activity
3.2. Antioxidant and Anti-Atherogenic Properties
3.3. Antihypertensive Potential
3.4. Antiarrhythmic Activity
4. Materials and Methods
- Articles including research articles, guidelines, monographs, technical papers, conference proceedings;
- Studies that are reported in the English language only,
- Treatment of cardiovascular diseases;
- Human studies, in vivo animal model, and in vitro laboratory studies;
- There was no limitation imposed on the year of publication of the studies.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 2018, 105, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Ohnuki, K.; Shimizu, K.; Kondo, R. (−)-Bornyl acetate induces autonomic relaxation and reduces arousal level after visual display terminal work without any influences of task performance in low-dose condition. Biomed. Res. 2011, 32, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Zhang, S.; Xie, Y.; Zhang, Z.; Zhao, W. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 2016, 11, 150–158. [Google Scholar] [CrossRef] [Green Version]
- de Lucena, J.D.; Gadelha-Filho, C.V.J.; da Costa, R.O.; de Araujo, D.P.; Lima, F.A.V.; Neves, K.R.T.; de Barros Viana, G.S. L-linalool exerts a neuroprotective action on hemiparkinsonian rats. Naunyn. Schmiedebergs Arch. Pharm. 2020, 393, 1077–1088. [Google Scholar] [CrossRef]
- Souto-Maior, F.N.; de Carvalho, F.L.; de Morais, L.C.; Netto, S.M.; de Sousa, D.P.; de Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharm. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Souto-Maior, F.N.; Fonseca, D.V.; Salgado, P.R.; Monte, L.O.; de Sousa, D.P.; de Almeida, R.N. Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm. Biol. 2017, 55, 63–67. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. alpha-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Khan, S.W.; Khatoon, S. Ethnobotanical studies on some useful herbs of Haramosh and Bugrote valleys in Gilgit, northern areas of Pakistan. Pak. J. Bot. 2008, 40, 43. [Google Scholar]
- Singletary, K. Coriander: Overview of potential health benefits. Nutr. Today 2016, 51, 151–161. [Google Scholar] [CrossRef]
- Mahendra, P.; Bisht, S. Coriandrum sativum: A daily use spice with great medicinal effect. Pharmacogn. J. 2011, 3, 84–88. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 25 September 2021).
- Rahman, F.A.; Abdullah, S.S.; Manan, W.; Tan, L.T.; Neoh, C.F.; Ming, L.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Salmasi, S.; et al. Efficacy and Safety of Cyclosporine in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Front. Pharm. 2018, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.N.; Liu, Z.H.; Zhao, Y.P.; Zhao, L.L.; Xue, T.K.; Lan, Q.K. Phytochemical and bioactive profile of Coriandrum sativum L. Food Chem. 2019, 286, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective potency of some spice herbs, a literature review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef]
- Sahib, N.G.; Anwar, F.; Gilani, A.H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals—A review. Phytother. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef] [Green Version]
- Chrysant, S.G.; Chrysant, G.S. Herbs Used for the Treatment of Hypertension and their Mechanism of Action. Curr. Hypertens. Rep. 2017, 19, 77. [Google Scholar] [CrossRef]
- Hussain, F.; Jahan, N.; Rahman, K.U.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their Angiotensin-Converting Enzyme (ACE) inhibition potential. Oxid. Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef] [Green Version]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharm. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Micallef, M.A.; Garg, M.L. Beyond blood lipids: Phytosterols, statins and omega-3 polyunsaturated fatty acid therapy for hyperlipidemia. J. Nutr. Biochem. 2009, 20, 927–939. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Ribeiro, G.H.M.; Rezende, L.F.; Fraga-Silva, R.A. Plant Terpenes on Treating Cardiovascular and Metabolic Disease: A Review. Protein Pept. Lett. 2021, 28, 750–760. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR J. Pharm. 2016, 6, 17–42. [Google Scholar] [CrossRef]
- Diederichsen, A. Coriander: Coriandrum sativum L.; Bioversity International: Rome, Italy, 1996; Volume 3. [Google Scholar]
- Nair, V.; Singh, S.; Gupta, Y.K. Anti-granuloma activity of Coriandrum sativum in experimental models. J. Ayurveda Integr. Med. 2013, 4, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Paniagua-Zambrana, N.Y.; Bussmann, R.W.; Romero, C. Coriandrum sativum L. A piaceae. Ethnobot. Andes 2020, 1–7. [Google Scholar] [CrossRef]
- Musselman, L.J. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics, 2nd ed.; Leung, A.T., Foster, S., Eds.; Springer: New York, NY, USA, 1996. [Google Scholar]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med. Res. 2004, 119, 167–179. [Google Scholar]
- Otoom, S.; Al-Safi, S.; Kerem, Z.; Alkofahi, A. The use of medicinal herbs by diabetic Jordanian patients. J. Herb. Pharmacother. 2006, 6, 31–41. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Rowais, N.A. Herbal medicine in the treatment of diabetes mellitus. Saudi Med. J. 2002, 23, 1327–1331. [Google Scholar] [PubMed]
- Chaudhry, N.; Tariq, P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak. J. Pharm. Sci. 2006, 19, 214–218. [Google Scholar]
- Ugulu, I.; Baslar, S.; Yorek, N.; Dogan, Y. The investigation and quantitative ethnobotanical evaluation of medicinal plants used around Izmir province, Turkey. J. Med. Plants Res. 2009, 3, 345–367. [Google Scholar]
- Emamghoreishi, M.; Heidari-Hamedani, G. Anticonvulsant effect of extract and essential oil of Coriandrum sativum seed in conscious mice. Iran. J. Pharm. Res. 2004, 3, 71. [Google Scholar]
- Emamghoreishi, M.; Heidari-Hamedani, G. Sedative-hypnotic activity of extracts and essential oil of Coriander seeds. Iran. J. Med. Sci. 2006, 31, 22–27. [Google Scholar]
- Aissaoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Acute diuretic effect of continuous intravenous infusion of an aqueous extract of Coriandrum sativum L. in anesthetized rats. J. Ethnopharmacol. 2008, 115, 89–95. [Google Scholar] [CrossRef]
- Taherian, A.A.; Vafaei, A.A.; Ameri, J. Opiate system mediate the antinociceptive effects of Coriandrum sativum in mice. Iran. J. Pharm. Res. 2012, 11, 679. [Google Scholar]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis; Wichtl, M., Ed.; Medpharm GmbH Scientific Publishers: Stuttgart, Germany, 2004. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Grieve, M. A Modern Herbal; Courier Corporation: North Chelmsford, MA, USA, 2013; Volume 2. [Google Scholar]
- Hassar, M. La phytothérapie au Maroc. Espérance Médicale 1999, 6, 83–85. [Google Scholar]
- Bashir, S.; Safdar, A. Coriander Seeds: Ethno-medicinal, Phytochemical and Pharmacological Profile. Sci. Spices Culin. Herbs-Latest Lab. Pre-Clin. Clin. Stud. 2020, 2, 39–64. [Google Scholar]
- Chauhan, P.; Jaryal, M.; Kumari, K.; Singh, M. Phytochemical and in vitro Antioxidant Potential of Aqueous Leaf Extracts of Brassica juncea and Coriandrum sativum; World Vegetable Center: Tainan, Taiwan, 2012. [Google Scholar]
- Ishikawa, T.; Kondo, K.; Kitajima, J. Water-soluble constituents of coriander. Chem. Pharm. Bull. 2003, 51, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Duenas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem. 2012, 132, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Oganesyan, E.; Nersesyan, Z.; Parkhomenko, A.Y. Chemical composition of the above-ground part of Coriandrum sativum. Pharm. Chem. J. 2007, 41, 149–153. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Babaei, S.; Mashayekhi, K.; Rokhzadi, A.; Amini, S. Anthocyanin content of coriander (Coriandrum sativum L.) leaves as affected by salicylic acid and nutrients application. IJB 2013, 3, 141–145. [Google Scholar] [CrossRef]
- Patel, D.K.; Desai, S.N.; Gandhi, H.P.; Devkar, R.V.; Ramachandran, A.V. Cardio protective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem. Toxicol. 2012, 50, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, Q.; Bashir, S.; Lyoussi, B.; Gilani, A.H. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J. Ethnopharmacol. 2009, 122, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.; Jahan, N.; ul-Rahman, K.; Khan, K.M.; Zafar, F. Anti-arrhythmic potential of Coriandrum sativum seeds in salt induced arrhythmic rats. Pak. Vet. J. 2016, 36, 465–471. [Google Scholar]
- Shrirame, B.; Geed, S.; Raj, A.; Prasad, S.; Rai, M.; Singh, A.; Singh, R.; Rai, B. Optimization of Supercritical Extraction of Coriander (Coriandrum sativum L.) Seed and Characterization of Essential Ingredients. J. Essent. Oil Bear. Plants 2018, 21, 330–344. [Google Scholar] [CrossRef]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira, J.M.; et al. Extraction of volatile oil from aromatic plants with supercritical carbon dioxide: Experiments and modeling. Molecules 2012, 17, 10550–10573. [Google Scholar] [CrossRef]
- Sourmaghi, M.H.; Kiaee, G.; Golfakhrabadi, F.; Jamalifar, H.; Khanavi, M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2015, 52, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Coşkuner, Y.; Karababa, E. Physical properties of coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 408–416. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem. Toxicol. 2009, 47, 22–34. [Google Scholar] [CrossRef]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Chahed, T.; Kchouk, M.E.; Marzouk, B. Changes on essential oil composition of coriander (Coriandrum sativum L.) fruits during three stages of maturity. Food Chem. 2007, 102, 1131–1134. [Google Scholar] [CrossRef]
- Zoubiri, S.; Baaliouamer, A. Essential oil composition of Coriandrum sativum seed cultivated in Algeria as food grains protectant. Food Chem. 2010, 122, 1226–1228. [Google Scholar] [CrossRef]
- Nejad Ebrahimi, S.; Hadian, J.; Ranjbar, H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010, 24, 1287–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, M.N.I.; Begum, J.; Sultana, M. Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh J. Pharmacol. 2009, 4, 150–153. [Google Scholar] [CrossRef]
- Eikani, M.H.; Golmohammad, F.; Rowshanzamir, S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 735–740. [Google Scholar] [CrossRef]
- Msaada, K.; Taârit, M.B.; Hosni, K.; Salem, N.; Tammar, S.; Bettaieb, I.; Hammami, M.; Limam, F.; Marzouk, B. Comparison of Different Extraction Methods for the Determination of Essential oils and Related Compounds from Coriander (Coriandrum sativum L.). Acta Chim. Slov. 2012, 59, 803–813. [Google Scholar]
- Benyoussef, E.H.; Saibi, S. Influence of essential oil composition on water distillation kinetics. Flavour Fragr. J. 2013, 28, 300–308. [Google Scholar] [CrossRef]
- HuaSong, S.; Yue, Z.; WenZhong, Z.; ShengQiang, D.; Kai, S.; ZhongXiao, T.; Yan, Y.; Ying, H. Changes of volatile components in Eryngium foetidum L. and Yunnan Coriandrum sativum L. with different plant organs. Food Res. Dev. 2016, 37, 161–165. [Google Scholar]
- Chung, I.-M.; Ahmad, A.; Kim, S.-J.; Naik, P.M.; Nagella, P. Composition of the essential oil constituents from leaves and stems of Korean Coriandrum sativum and their immunotoxicity activity on the Aedes aegypti L. Immunopharmacol. Immunotoxicol. 2012, 34, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Arak, E.; Raal, A. Essential oil composition of Coriandrum sativum L. fruits from different countries. J. Essent. Oil Bear. Plants 2011, 14, 118–123. [Google Scholar] [CrossRef]
- Gil, A.; De La Fuente, E.B.; Lenardis, A.E.; López Pereira, M.; Suárez, S.A.; Bandoni, A.; Van Baren, C.; Di Leo Lira, P.; Ghersa, C.M. Coriander essential oil composition from two genotypes grown in different environmental conditions. J. Agric. Food Chem. 2002, 50, 2870–2877. [Google Scholar] [CrossRef]
- Saygi, K.O. Quantification of Phenolics from Coriandrum sativum vulgare and Coriandrum sativum microcarpum by HPLC–DAD. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 1319–1326. [Google Scholar] [CrossRef]
- Divya, P.; Puthusseri, B.; Neelwarne, B. Carotenoid content, its stability during drying and the antioxidant activity of commercial coriander (Coriandrum sativum L.) varieties. Food Res. Int. 2012, 45, 342–350. [Google Scholar] [CrossRef]
- Sriti, J.; Wannes, W.A.; Talou, T.; Mhamdi, B.; Hamdaoui, G.; Marzouk, B. Lipid, fatty acid and tocol distribution of coriander fruit’s different parts. Ind. Crops Prod. 2010, 31, 294–300. [Google Scholar] [CrossRef]
- Sriti, J.; Talou, T.; Wannes, W.A.; Cerny, M.; Marzouk, B. Essential oil, fatty acid and sterol composition of Tunisian coriander fruit different parts. J. Sci. Food Agric. 2009, 89, 1659–1664. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food. Plants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Kajal, A.; Singh, R. Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS ONE 2019, 14, e0213147. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaur, N.; Kishore, L.; Gupta, G.K. Management of diabetic complications: A chemical constituents based approach. J. Ethnopharmacol. 2013, 150, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Cha, J.M.; Yoon, D.; Kim, S.Y.; Kim, C.S.; Lee, K.R. Neurotrophic and anti-neuroinflammatory constituents from the aerial parts of Coriandrum sativum. Bioorganic Chem. 2020, 105, 104443. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Amer, M.M.A.; Awad, A.E.S. Coriander (Coriandrum sativum L.) seed oil improves plasma lipid profile in rats fed a diet containing cholesterol. Eur. Food Res. Technol. 2008, 227, 1173–1182. [Google Scholar] [CrossRef]
- Ananthan, R.; Latha, M.; Ramkumar, K.; Pari, L.; Baskar, C.; Bai, V.N. Modulatory effects of Gymnema montanum leaf extract on alloxan-induced oxidative stress in Wistar rats. Nutrition 2004, 20, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, P.; Jasuja, N.D.; Joshi, S.C. Ameliorative efficiency of Coriandrum sativum seed extract on atherosclerosis and oxidative stress in male albino hyperlipidemic rabbits. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 26–39. [Google Scholar]
- Lal, A.A.; Kumar, T.; Murthy, P.B.; Pillai, K.S. Hypolipidemic effect of Coriandrum sativum L. in triton-induced hyperlipidemic rats. Indian J. Exp. Biol. 2004, 42, 909–912. [Google Scholar] [PubMed]
- Zeb, F.; Safdar, M.; Fatima, S.; Khan, S.; Alam, S.; Muhammad, M.; Syed, A.; Habib, F.; Shakoor, H. Supplementation of garlic and coriander seed powder: Impact on body mass index, lipid profile and blood pressure of hyperlipidemic patients. Pak. J. Pharm. Sci. 2018, 31, 1935–1941. [Google Scholar]
- Rajeshwari, U.; Shobha, I.; Andallu, B. Comparison of aniseeds and coriander seeds for antidiabetic, hypolipidemic and antioxidant activities. Spatula DD Peer Rev. J. Complement. Med. Drug Discov. 2011, 1, 9–16. [Google Scholar] [CrossRef]
- Aissaoui, A.; Zizi, S.; Israili, Z.H.; Lyoussi, B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J. Ethnopharmacol. 2011, 137, 652–661. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Maity, N.; Nema, N.K.; Bhadra, S.; Saha, B.P.; Mukherjee, P.K. Angiotensin converting enzyme inhibition activity of fennel and coriander oils from India. Nat. Prod. Commun. 2013, 8, 671–672. [Google Scholar] [CrossRef] [Green Version]
- Afsheen, N.; Khalil Ur, R.; Jahan, N.; Khan, K.M.; Zia, M.A. Optimization of cardioprotective potential of various concentrations of medicinal plants by using response surface methodology. Pak. Vet. J. 2019, 39, 13–18. [Google Scholar] [CrossRef]
- Dhanapakiam, P.; Joseph, J.M.; Ramaswamy, V.K.; Moorthi, M.; Kumar, A.S. The cholesterol lowering property of coriander seeds (Coriandrum sativum): Mechanism of action. J. Environ. Biol. 2008, 29, 53–56. [Google Scholar]
- Dhyani, N.; Parveen, A.; Siddiqi, A.; Hussain, M.E.; Fahim, M. Cardioprotective Efficacy of Coriandrum sativum (L.) Seed Extract in Heart Failure Rats Through Modulation of Endothelin Receptors and Antioxidant Potential. J. Diet. Suppl. 2020, 17, 13–26. [Google Scholar] [CrossRef]
- Chithra, V.; Leelamma, S. Hypolipidemic effect of coriander seeds (Coriandrum sativum): Mechanism of action. Plant Foods Hum. Nutr. 1997, 51, 167–172. [Google Scholar] [CrossRef]
- Patel, D.; Desai, S.; Gajaria, T.; Devkar, R.; Ramachandran, A.V. Coriandrum sativum L. seed extract mitigates lipotoxicity in raw 264.7 cells and prevents atherogenic changes in rats. EXCLI J. 2013, 12, 313–334. [Google Scholar]
- Kousar, S.; Jahan, N.; Khalil Ur, R.; Nosheen, S. Antilipidemic activity of Coriandrum sativum. Biosci. Res. 2011, 8, 8–14. [Google Scholar]
- Wang, X.; Zhu, C.; Peng, T.; Zhang, W.; Zhang, J.; Liu, H.; Wu, C.; Pan, X.; Wu, C. Enhanced stability of an emulsion enriched in unsaturated fatty acids by dual natural antioxidants fortified in both the aqueous and oil phases. Food Hydrocoll. 2018, 82, 322–328. [Google Scholar] [CrossRef]
- Froyen, E. The effects of fat consumption on low-density lipoprotein particle size in healthy individuals: A narrative review. Lipids Health Dis. 2021, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Bays, H. Clinical overview of Omacor: A concentrated formulation of omega-3 polyunsaturated fatty acids. Am. J. Cardiol. 2006, 98, 71i–76i. [Google Scholar] [CrossRef] [PubMed]
- Colin, A.; Reggers, J.; Castronovo, V.; Ansseau, M. Lipids, depression and suicide. Encephale 2003, 29, 49–58. [Google Scholar]
- Bao, L.; Hu, L.; Zhang, Y.; Wang, Y.I. Hypolipidemic effects of flavonoids extracted from Lomatogonium rotatum. Exp. Med. 2016, 11, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Plösch, T.; Kruit, J.K.; Bloks, V.W.; Huijkman, N.C.; Havinga, R.; Duchateau, G.S.; Lin, Y.; Kuipers, F. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the Abcg5/8 transporter. J. Nutr. 2006, 136, 2135–2140. [Google Scholar] [CrossRef]
- Hwang, G.-H.; Heo, Y.-R.; Lee, H.-J.; Park, O.-J.; Kang, S.-K.; Kim, Y.-D. Effects of Coriandrum sativum L. on lipid metabolism in rats with hypertriglyceridemic diet. Nutr. Sci. 2001, 4, 13–19. [Google Scholar]
- Sriti, J.; Wannes, W.A.; Talou, T.; Mhamdi, B.; Cerny, M.; Marzouk, B. Lipid profiles of Tunisian coriander (Coriandrum sativum) seed. J. Am. Oil Chem. Soc. 2010, 87, 395–400. [Google Scholar] [CrossRef]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef]
- Kong, C.K.; Low, L.E.; Siew, W.S.; Yap, W.H.; Khaw, K.Y.; Ming, L.C.; Mocan, A.; Goh, B.H.; Goh, P.H. Biological Activities of Snowdrop (Galanthus spp., Family Amaryllidaceae). Front. Pharm. 2020, 11, 552453. [Google Scholar] [CrossRef]
- Anmol, R.J.; Marium, S.; Hiew, F.T.; Han, W.C.; Kwan, L.K.; Wong, A.K.Y.; Khan, F.; Sarker, M.M.R.; Chan, S.Y.; Kifli, N.; et al. Phytochemical and Therapeutic Potential of Citrus grandis (L.) Osbeck: A Review. J. Evid. Based Integr. Med. 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Greaves, D.R.; Gough, P.J.; Gordon, S. Recent progress in defining the role of scavenger receptors in lipid transport, atherosclerosis and host defence. Curr. Opin. Lipidol. 1998, 9, 425–432. [Google Scholar] [CrossRef]
- Björkerud, B.; Björkerud, S.r. Contrary effects of lightly and strongly oxidized LDL with potent promotion of growth versus apoptosis on arterial smooth muscle cells, macrophages, and fibroblasts. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 416–424. [Google Scholar] [CrossRef]
- Sreelatha, S.; Inbavalli, R. Antioxidant, antihyperglycemic, and antihyperlipidemic effects of Coriandrum sativum leaf and stem in alloxan-induced diabetic rats. J. Food Sci. 2012, 77, T119–T123. [Google Scholar] [CrossRef]
- Dianat, M.; Negin, A.; Mohammad, B.; Yaghoub, F. Antidysrhythmic, inotropic and chronotropic effects of ellagic acid and forced exercise in rat. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1549–1557. [Google Scholar]
- Ramadan, M.F.; Moersel, J.-T. Screening of the antiradical action of vegetable oils. J. Food Compos. Anal. 2006, 19, 838–842. [Google Scholar] [CrossRef]
- Potter, T.L. Essential oil composition of cilantro. J. Agric. Food Chem. 1996, 44, 1824–1826. [Google Scholar] [CrossRef]
- Abiko, Y.; Kumagai, Y. Preventive Agents and Phytochemicals for Reducing the Adverse Health Effects of Arsenic. Arsen. Contam. Asia 2019, 151–161. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Ali, F.E.; Hassanein, E.H.; El-Bahrawy, A.H.; Omar, Z.M.; Rashwan, E.K.; Abdel-Wahab, B.A.; Abd-Elhamid, T.H. Nephroprotective effect of umbelliferone against cisplatin-induced kidney damage is mediated by regulation of NRF2, cytoglobin, SIRT1/FOXO-3, and NF-kB-p65 signaling pathways. J. Biochem. Mol. Toxicol. 2021, 35, e22738. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol. 2012, 8, 863–884. [Google Scholar] [CrossRef] [Green Version]
- Nwagha, U.; Ikekpeazu, E.; Ejezie, F.; Neboh, E.; Maduka, I. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar] [PubMed]
- Boers, M.; Nurmohamed, M.T.; Doelman, C.J.; Lard, L.R.; Verhoeven, A.C.; Voskuyl, A.E.; Huizinga, T.W.; van de Stadt, R.J.; Dijkmans, B.A.; van der Linden, S. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 842–845. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, A.N.; Papavasiliou, E.C.; Lourida, E.S.; Alamanos, Y.; Kostara, C.; Tselepis, A.D.; Drosos, A.A. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: Effect of early treatment—A prospective, controlled study. Arthritis Res. Ther. 2006, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Nazni, P.; Dharmaligam, R. Isolation and separation of phenolic compound from coriander flowers. Int. J. Agric. Food Sci. 2014, 4, 13–21. [Google Scholar]
- Rajeshwari, C.; Andallu, B. Isolation and simultaneous detection of flavonoids in the methanolic and ethanolic extracts of Coriandrum sativum L. seeds by RP-HPLC. Pak. J. Food Sci. 2011, 21, 13–21. [Google Scholar]
- Msaada, K.; Jemia, M.B.; Salem, N.; Bachrouch, O.; Sriti, J.; Tammar, S.; Bettaieb, I.; Jabri, I.; Kefi, S.; Limam, F. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arab. J. Chem. 2017, 10, S3176–S3183. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, C.; Liu, Q.; Ou, S.; Zhang, L.; Peng, X. Combinative effect of sardine peptides and quercetin alleviates hypertension through inhibition of angiotensin I converting enzyme activity and inflammation. Food Res. Int. 2017, 100, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O.; Ayodeji, F. Ameliorative effect of Rutin on sodium fluoride-induced hypertension through modulation of Kim-1/NF-κB/Nrf2 signaling pathway in rats. Environ. Toxicol. 2018, 33, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-L.; Yu, X.-J.; Hu, H.-B.; Yang, Q.-W.; Liu, K.-L.; Chen, Y.-M.; Zhang, Y.; Zhang, D.-D.; Tian, H.; Zhu, G.-Q. Apigenin Improves Hypertension and Cardiac Hypertrophy Through Modulating NADPH Oxidase-Dependent ROS Generation and Cytokines in Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 721–736. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O.; Saba, A.B.; et al. Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. Biofactors 2018, 44, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb)(Simaroubaceae). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 32–36. [Google Scholar]
- Chen, C.-H.; Lin, J.-Y.; Lin, C.-N.; Hsu, S.-Y. Inhibition of angiotensin-I-converting enzyme by tetrahydroxyxanthones isolated from Tripterospermum lanceolatum. J. Nat. Prod. 1992, 55, 691–695. [Google Scholar] [CrossRef]
- Bormann, H.; Melzig, M. Inhibition of metallopeptidases by flavonoids and related compounds. Die Pharm. 2000, 55, 129–132. [Google Scholar]
- Lacaille-Dubois, M.; Franck, U.; Wagner, H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M. Calcium Antagonists in Clinical Medicine; Hanley Belfus Inc.: Phila, PA, USA, 1992; Volume 183. [Google Scholar]
- Chiu, A.T.; McCall, D.E.; Timmermans, P.B. Pharmacological characteristics of receptor-operated and potential-operated Ca2+ channels in rat aorta. Eur. J. Pharmacol. 1986, 127, 1–8. [Google Scholar] [CrossRef]
- Mendel, B.; Christianto; Setiawan, M.; Prakoso, R.; Siagian, S.N. A Comparative Effectiveness Systematic Review and Meta-Analysis of Drugs for the Prophylaxis of Junctional Ectopic Tachycardia. Curr. Cardiol. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]





| Scientific Name: | Coriandrum sativum |
|---|---|
| Common names: | Coriander, Chinese Parsley, cilantro, kusthumbari, dhanya, dhane, pak chee, yuan sui, hu sui |
| Kingdom: | Plantae |
| Subkingdom: | Tracheobionta |
| Superdivision: | Spermatophyta |
| Division: | Magnoliophyta |
| Class: | Magnoliopsida |
| Subclass: | Rosidae |
| Order: | Apiales |
| Family: | Apiaceae/Umbelliferae |
| Genus: | Coriandrum L. |
| Species: | Coriandrum sativum L. |
| Traditional Uses | Area | Plant Parts Used | Reference |
|---|---|---|---|
| Rheumatoid arthritis, inflammation, and joint pain | India | Seeds/seeds aqueous extract | [26,38,39] |
| For measles, diabetes, aerophagy, gastroenteritis | China | The whole plant parts | [40] |
| Antiviral and neuro-energizer | Pakistani herbal drugs (Intellan) | Aerial parts of the plant | |
| Some liver diseases | - | Aqueous extract of the roasted seeds | |
| Carminative, diuretic, dyspeptic complaints, loss of appetite, convulsion, insomnia, and anxiety and in medical purposes | Iranian traditional medicine | Powdered seeds or dry extract | [38] |
| Diaphoretic, diuretic, carminative, and stimulant activity | Iranian traditional medicine | The whole plant parts | [37,41] |
| Diuretic and for some renal diseases | Morocco | Oral administration of plant parts | [42] |
| Mouth ulcer and eye redness | - | leaves decoction | [27] |
| Grounded as an ingredient of curry powder and gingerbread, also a component of liquesces and spirits. Aromatic ingredient of tobacco and perfumes. In Unani medicine to quench thirst and for melancholia. | India | Seeds and aqueous infusion of leaves | [39] |
| Stimulant and carminative; stomachic, antibilious, digestive stimulant | India | Leaves | [29] |
| Lower blood glucose levels | Saudi Arabia, Jordan, Morocco | Fruits, decoction of leaves and seeds | [30,31,32] |
| Aphrodisiac, analgesic, antimicrobial properties | - | The volatile oil | [33] |
| Appetizer, Digestive, Carminative | Turkey | Infusion of the seeds | [34] |
| For anxiety, sedative and muscle relaxant effect | - | The aqueous extract | [35,36] |
| Treats Influenza, bad breath, unpleasant odor from genitalia | Traditional Chinese Medicine | Seeds | [43] |
| Against worm and to treat rheumatism | The European pharmacopeia | Fruits | |
| Stimulates gastric secretion, treats gastric ulcers and mouth infections | Asian region | Essential oils |
| Plant Part | Extraction Method | Dosage Employed | Phytochemicals Characterized | Isolation Method | Experimental Subject | Cardiovascular Effects (Outcome) | Reference |
|---|---|---|---|---|---|---|---|
| Leaves and stem | Ethanol extraction | 200 mg/kg | Phenolics, flavonoids | Evaporation | Wistar albino rats | Antioxidant, Hypolipidemic Normoglycemic | Ananthan, et al. [78] |
| Seeds | Methanol extraction | 100 mg/kg, 200 mg/kg, 300 mg/kg | N/D | Reduced pressure | Adult male Wistar rats | Decrease cardiac damage that causes myocardial infarction | Patel, Desai, Gandhi, Devkar and Ramachandran [49] |
| Seeds | Soxhlet extraction | 250 mg/kg | N/D | Reduced pressure | Male albino rabbits | Hypolipidemic | Sharma, et al. [79] |
| Market-procured coriander powder suspended in water | - | 1 g/kg | N/D | - | Male Wistar rats | Hypolipidemic | Lal, et al. [80] |
| Seed powder | - | 2 g per day | N/D | - | Human individuals | Lower elevated blood pressure, Hypocholesterolemic | Zeb, et al. [81] |
| Market-procured seeds, powdered | - | 5 g per day | N/D | - | Type 2 diabetic patients | Hypolipidemic, antioxidant | Rajeshwari, et al. [82] |
| Seeds | Aqueous extraction | 200 mg/kg | N/D | - | Meriones shawi rats | Hypolipidemic Normo-glycemic Cardioprotective | Aissaoui, et al. [83] |
| Seeds | Hydrodistillation | IC50: 34.8 ± 2.3 μg/mL | Linalool | Hydrodistillation | In vitro study | Antihypertensive, Antioxidant | Chaudhary, et al. [84] |
| Seeds | Maceration in methanol | 183 mg/kg | N/D | - | Rats | Cardioprotective | Afsheen, et al. [85] |
| Seeds | Homogenized seeds | 10% of diet | N/D | - | Sprague-Dawley rats | Hypolipidemic | Dhanapakiam, et al. [86] |
| Seeds | Aqueous extraction | 1 g/kg | N/D | - | Wistar albino rats | Cardioprotective, Hypolipidemic, Antioxidant, Improved left ventricle functions | Dhyani, et al. [87] |
| Leaves | Soxhlet extraction with multiple solvents | IC50: 28.91 ± 13.42 μg/mL | Pinocembrin, Apigenin, pseudobaptigenin, galangin-5-methyl ether, quercetin, baicalein, kaempferol, pinobanksin-glycosides, rutin, isorhamnetin, daidzein, luteolin, pectolinarigenin | LC-ESI-MS/MS | In vitro study | Antihypertensive | Hussain, Jahan, Rahman, Sultana and Jamil [20] |
| Seeds | Solvent extraction | Addition of 100 g to diet | Stigmasterol, Lanosterol, β-Sitosterol, D5Avenasterol, Sitostanol, D7stigmastenol, D7 Avenasterol, Tocopherols | Gas-liquid chromatography | Male albino rats | Hypocholesterolemic effect | Ramadan, Amer and Awad [77] |
| Seeds | Homogenized seeds | 10% of diet | N/D | - | Female albino rats | Hypolipidemic | Chithra and Leelamma [88] |
| Seeds | Water extraction | 300 mg/kg | N/D | - | Male albino rats | Antiarrhythmic | Rehman, Jahan, Khalil ul, Khan and Zafar [51] |
| Seeds | Maceration with Aqueous/Methanol | 200 mg/kg | N/D | - | In vitro and in vivo study on Sprague-Dawley rats | Antioxidant, Hypocholesterolemic Anti-atherogenic | Patel, et al. [89] |
| Fruit | Maceration with Aqueous/Methanol | 1–30 mg/mL as hypotensive 1–10 mg/kg as diuretic | N/D | Organic fractionation of the crude extract | In vivo and in vitro study | Anti-hypertensive, Diuretic | Jabeen, Bashir, Lyoussi and Gilani [50] |
| Leaves | Maceration with Methanol | 100 mg/kg | N/D | - | Rabbits | Hypolipidemic | Kousar, et al. [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S.; et al. Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules 2022, 27, 209. https://doi.org/10.3390/molecules27010209
Mahleyuddin NN, Moshawih S, Ming LC, Zulkifly HH, Kifli N, Loy MJ, Sarker MMR, Al-Worafi YM, Goh BH, Thuraisingam S, et al. Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules. 2022; 27(1):209. https://doi.org/10.3390/molecules27010209
Chicago/Turabian StyleMahleyuddin, Nisa Najibah, Said Moshawih, Long Chiau Ming, Hanis Hanum Zulkifly, Nurolaini Kifli, Mei Jun Loy, Md. Moklesur Rahman Sarker, Yaser Mohammed Al-Worafi, Bey Hing Goh, Shobna Thuraisingam, and et al. 2022. "Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits" Molecules 27, no. 1: 209. https://doi.org/10.3390/molecules27010209
APA StyleMahleyuddin, N. N., Moshawih, S., Ming, L. C., Zulkifly, H. H., Kifli, N., Loy, M. J., Sarker, M. M. R., Al-Worafi, Y. M., Goh, B. H., Thuraisingam, S., & Goh, H. P. (2022). Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules, 27(1), 209. https://doi.org/10.3390/molecules27010209