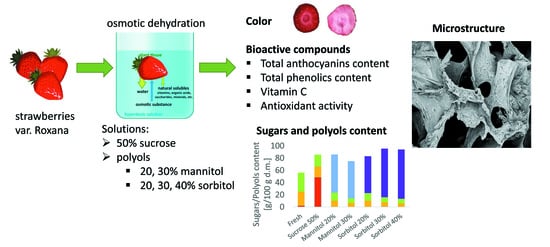
The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Different Concentrations of Polyols on Osmotic Dehydration Process of Organic Strawberries
2.2. Effect of Different Concentrations of Polyols on Colour Parameters of Organic Strawberries
2.3. Effect of Different Concentrations of Polyols on Bioactive Compound Contents in Organic Strawberries
2.3.1. Total Anthocyanin Content (TAC) in Organic Strawberries Subjected to Osmotic Dehydration in Sucrose and Polyol Solutions
2.3.2. Total Phenolics Content in Organic Strawberries Subjected to Osmotic Dehydration in Sucrose and Polyol Solutions
2.3.3. Vitamin C Content in Organic Strawberries Subjected to Osmotic Dehydration in Sucrose and Polyol Solutions
2.3.4. Antioxidant Activity of Organic Strawberries Subjected to Osmotic Dehydration in Sucrose and Polyol Solutions
2.4. Effect of Different Concentrations of Polyols on Sugars and Polyol Content in Organic Strawberries
2.5. Effect of Different Concentrations of Polyols on Organic Strawberry Structure
2.6. Cluster Analysis (CA)
3. Materials and Methods
3.1. Materials
3.2. Experimental Design
3.3. Osmotic Dehydration Process in Sucrose and Polyols
3.4. Physical and Chemical Properties of Organic Strawberries Subjected to Osmotic Dehydration in Sucrose and Polyol Solutions
3.4.1. Dry Matter Content in Organic Strawberries Subjected to Osmotic Dehydration
3.4.2. Colour Parameters of Organic Strawberries Subjected to Osmotic Dehydration
3.4.3. Bioactive Compounds in Organic Strawberries Subjected to Osmotic Dehydration
Extraction Procedure
Total Anthocyanin Content of Organic Strawberries Subjected to Osmotic Dehydration
- A = (A510–A700) pH1 − (A510–A700) pH4.5;
- MW—molecular weight of cyanide 3-glucoside (449.2 g/mol);
- DF—sample dilution factor;
- ϵ—molar absorption coefficient of cyanidin-3-glucoside (26 900 L/mol×cm);
- L—optical path length for the solution in the well (0.173 cm).
Total Phenolics Content in Organic Strawberries Subjected to Osmotic Dehydration
Vitamin C Content in Organic Strawberries Subjected to Osmotic Dehydration
Antioxidant Activity of Organic Strawberries Subjected to Osmotic Dehydration
Determination of the Antioxidant Capacity against DPPH and ABTS Radicals
Reducing Power (RP)
3.4.4. Sugar and Polyol Content in Organic Strawberries Subjected to Osmotic Dehydration
3.4.5. Scanning Electron Microscopy Analysis (SEM)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, C.; Wu, H.; Masisi, K.; Malunga, L.N.; Song, Y. Strawberries. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: San Diego, CA, USA, 2020; Volume 2, pp. 423–435. ISBN 9780128127803. [Google Scholar]
- FAOSTAT. Statistic Database. 2019. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 8 December 2021).
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Porras, C.; Saavedra-Leos, M.Z.; López-Martinez, L.A.; Espinosa-Solis, V.; Terán-Figueroa, Y.; Toxqui-Terán, A.; Compeán-Martínez, I. Strawberry juice powders: Effect of spray-drying conditions on the microencapsulation of bioactive components and physicochemical properties. Molecules 2021, 26, 5466. [Google Scholar] [CrossRef] [PubMed]
- Tylewicz, U.; Oliveira, G.; Alminger, M.; Nohynek, L.; Dalla Rosa, M.; Romani, S. Antioxidant and antimicrobial properties of organic fruits subjected to PEF-assisted osmotic dehydration. Innov. Food Sci. Emerg. Technol. 2020, 62, 102341. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Lenart, A. Osmotic dehydration of fruits and vegetables. In Handbook of Industrial Drying; Mujumdar, A., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 665–688. ISBN 9780128033654. [Google Scholar]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Akbarian, M.; Ghasemkhani, N.; Moayedi, F. Osmotic dehydration of fruits in food industrial: A review. J. Food Sci. Technol. 2014, 4, 42–57. [Google Scholar] [CrossRef]
- Ramya, V.; Jain, N.K. A Review on Osmotic Dehydration of Fruits and Vegetables: An Integrated Approach. J. Food Process Eng. 2017, 40, e12440. [Google Scholar] [CrossRef]
- Gribova, N.A.; Perov, V.I.; Eliseeva, L.G.; Berketova, L.V.; Nikolayeva, M.A.; Soltaeva, N.L. Innovative technology of processing berries by osmotic dehydration. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012119. [Google Scholar] [CrossRef]
- Cichowska, J.; Żubernik, J.; Czyżewski, J.; Kowalska, H.; Witrowa-Rajchert, D. Efficiency of osmotic dehydration of apples in polyols solutions. Molecules 2018, 23, 446. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, L.; Jemai, A.B.; Vorobiev, E. Osmotic dehydration of some agro-food tissue pre-treated by pulsed electric field: Impact of impeller’s Reynolds number on mass transfer and color. J. King. Saud. Univ. Eng. Sci. 2014, 26, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Kumari, D. Recent Development in Osmotic Dehydration of Fruit and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 552–561. [Google Scholar] [CrossRef]
- Porciuncula, B.D.A.; Zotarelli, M.F.; Carciofi, B.A.M.; Laurindo, J.B. Determining the effective diffusion coefficient of water in banana (Prata variety) during osmotic dehydration and its use in predictive models. J. Food Eng. 2013, 119, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Dermesonlouoglou, E.; Paraskevopoulou, E.; Andreou, V.; Taoukis, P. Osmotic dehydration for the production of novel pumpkin cut products of enhanced nutritional value and sustainability. Appl. Sci. 2020, 10, 6225. [Google Scholar] [CrossRef]
- Samborska, K.; Eliasson, L.; Marzec, A.; Kowalska, J.; Piotrowski, D.; Lenart, A.; Kowalska, H. The effect of adding berry fruit juice concentrates and by-product extract to sugar solution on osmotic dehydration and sensory properties of apples. J. Food Sci. Technol. 2019, 56, 1927–1938. [Google Scholar] [CrossRef] [Green Version]
- Lech, K.; Michalska, A.; Wojdylo, A.; Nowicka, P.; Figiel, A. The influence of the osmotic dehydration process on physicochemical properties of osmotic solution. Molecules 2017, 22, 2246. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, H.; Kowalska, J.; Masiarz, E.; Maziarz, S.; Pochitskaya, I. The use of apple and beetroot juices to osmotic dehydration of apples. Postępy Tech. Przetwórstwa Spożywczego 2020, 1, 51–57. [Google Scholar]
- Mendonça, K.S.; de Jesus, J.R.; Pereira, M.C.A.; Corrêa, J.L.G. Osmotic Dehydration of Yacon Slices: Effect of Different Polyols on Mass Transfer Parameters. Blucher Chem. Eng. Proc. 2015, 1, 5069–5076. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Latocha, P.; Gondek, E. Mass transfer in osmotic dehydration of kiwiberry: Experimental and mathematical modelling studies. Molecules 2018, 23, 1236. [Google Scholar] [CrossRef]
- Cichowska, J.; Woźniak, Ł.; Figiel, A.; Witrowa-Rajchert, D. The influence of osmotic dehydration in polyols solutions on sugar profiles and color changes of apple tissue. Period. Polytech. Chem. Eng. 2020, 64, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, M.; Fijalkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of ultrasound treatment during osmotic dehydration on bioactive compounds of cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Witrowa-Rajchert, D.; Gondek, E. The Influence of Osmotic Dehydration Conditions on Drying Kinetics and Total Carotenoid Content of Kiwiberry (Actinidia arguta). Int. J. Food Eng. 2020, 16, 1–8. [Google Scholar] [CrossRef]
- Grembecka, M. Sugar alcohols—Their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Grembecka, M. Sugar alcohols. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–275. ISBN 9780128140451. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation (ID 463, 464, 563, 618, 647, 1182, 1591, 2907, 2921, 4300), and reduction of post-prandial glycaemic responses (ID 617, 619, 669, 1590, 1762, 2903, 2908, 2920) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2076. [Google Scholar] [CrossRef]
- European Parliament; The Concil of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0016:0033:en:PDF (accessed on 20 December 2021).
- Cichowska, J.; Witrowa-Rajchert, D.; Stasiak-Różanska, L.; Figiel, A. Ultrasound-assisted osmotic dehydration of apples in polyols and dihydroxyacetone (DHA) solutions. Molecules 2019, 24, 3429. [Google Scholar] [CrossRef] [Green Version]
- Cichowska-Bogusz, J.; Figiel, A.; Carbonell-Barrachina, A.A.; Pasławska, M.; Witrowa-Rajchert, D. Physicochemical properties of dried apple slices: Impact of osmo-dehydration, sonication, and drying methods. Molecules 2020, 25, 1078. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, H.; Woźniak, Ł.; Masiarz, E.; Stelmach, A.; Salamon, A.; Kowalska, J.; Piotrowski, D.; Marzec, A. The impact of using polyols as osmotic agents on mass exchange during osmotic dehydration and their content in osmodehydrated and dried apples. Dry. Technol. 2020, 38, 1620–1631. [Google Scholar] [CrossRef]
- Mendonça, K.; Correa, J.; Junqueira, J.; Angelis-Pereira, M.; Cirillo, M. Mass Transfer Kinetics of the Osmotic Dehydration of Yacon Slices with Polyols. J. Food Process. Preserv. 2017, 41, e12983. [Google Scholar] [CrossRef] [Green Version]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Mathematical Modelling of Osmotic Dehydration Kinetics of Apple Cubes. J. Food Process. Preserv. 2017, 41, e12895. [Google Scholar] [CrossRef]
- Brochier, B.; Marczak, L.D.F.; Noreña, C.P.Z. Osmotic Dehydration of Yacon Using Glycerol and Sorbitol as Solutes: Water Effective Diffusivity Evaluation. Food Bioprocess Technol. 2015, 8, 623–636. [Google Scholar] [CrossRef]
- Yang, D.H.; Li, X.Y.; Zhao, Y.F.; Hou, Q.M.; Meng, Q.Y.; Li, L. Osmotic dehydration kinetics of fresh and frozen blueberries considering volume shrinkage in a novel ternary solution. Food Sci. Technol. 2021, 41, 790–798. [Google Scholar] [CrossRef]
- Deis, R.C.; Kearsley, M.W. Sorbitol and mannitol. In Sweeteners and Sugar Alternatives in Food Technology; O’Donnell, K., Kearsley, M.W., Eds.; Wiley-Blackwell: West Sussex, UK, 2012; pp. 331–346. ISBN 9781439846155. [Google Scholar]
- Tylewicz, U.; Nowacka, M.; Martín-García, B.; Wiktor, A.; Gómez Caravaca, A.M. Target sources of polyphenols in different food products and their processing by-products. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 135–176. [Google Scholar]
- Luo, H.; Dai, C.; Li, Y.; Feng, J.; Liu, Z.; Kang, C. Reduced Anthocyanins in Petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J. Exp. Bot. 2018, 69, 2595–2608. [Google Scholar] [CrossRef] [Green Version]
- Karaaslan, N.M.; Yaman, M. Anthocyanin profile of strawberry fruit as affected by extraction conditions. Int. J. Food Prop. 2018, 20, S2313–S2322. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, M.; Wiktor, A.; Dadan, M.; Rybak, K.; Anuszewska, A.; Materek, L.; Witrowa-Rajchert, D. The application of combined pre-treatment with utilization of sonication and reduced pressure to accelerate the osmotic dehydration process and modify the selected properties of cranberries. Foods 2019, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Horie, A.; Nakabayashi, M.; Nishimura, K.; Yasunobu, T. Influence of processing conditions of atmospheric freeze-drying/low-temperature drying on the drying kinetics of sliced fruits and their vitamin C retention. J. Agric. Food Res. 2021, 6, 100231. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of healthy snack based on kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32011R1169&from=EN (accessed on 28 January 2022).
- Tiroutchelvame, D.; Sivakumar, V.; Prakash Maran, J. Mass transfer kinetics during osmotic dehydration of amla (Emblica officinalis L.) cubes in sugar solution. Chem. Ind. Chem. Eng. Q. 2015, 21, 547–559. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Mass Transfer in Osmotic Dehydration of Food Products: Comparison Between Mathematical Models. Food Eng. Rev. 2016, 8, 116–133. [Google Scholar] [CrossRef]
- Brochier, B.; Marczak, L.D.F.; Noreña, C.P.Z. Use of different kinds of solutes alternative to sucrose in osmotic dehydration of yacon. Braz. Arch. Biol. Technol. 2015, 58, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Dermesonlouoglou, E.K.; Giannakourou, M.C. Modelling dehydration of apricot in a non-conventional multi-component osmotic solution: Effect on mass transfer kinetics and quality characteristics. J. Food Sci. Technol. 2018, 55, 4079–4089. [Google Scholar] [CrossRef]
- Macedo, L.L.; Corrêa, J.L.G.; Petri Júnior, I.; da Silva Araújo, C.; Vimercati, W.C. Intermittent microwave drying and heated air drying of fresh and isomaltulose (Palatinose) impregnated strawberry. LWT 2022, 155, 112918. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Corrêa, J.L.G.; Botrel, D.A.; Vilela, M.B.; Batista, L.R.; Freire, L. Reuse of sorbitol solution in pulsed vacuum osmotic dehydration of yacon (Smallanthus sonchifolius). J. Food Process. Preserv. 2017, 41, e13306. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, W.; Mezrigui, S.; Badwaik, L.S.; Bejar, A.K.; Perez, C.T.; Kechaou, N. Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason. Sonochem. 2017, 36, 286–300. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of Osmotic Dehydration (with and without Sonication) and Pectin-Based Coating Pretreatments on Functional Properties and Color of Hot-Air Dried Apricot Cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Hui, L.C.; Sulaiman, R.; Shamsudin, R.; Rukayadi, Y. Effect of storage on physicochemical properties and microbiological stability of osmodehydrated pineapple (Ananas comosus) treated with sucrose-sorbitol mixtures. Res. J. Pharm. Biol. Chem. Sci. 2017, 2, 296–307. [Google Scholar]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Ciurzyńska, A.; Czajkowska, K.; Cichowska, J.; Rybak, K.; Lenart, A. Osmotic dehydration of Honeoye strawberries in solutions enriched with natural bioactive molecules. LWT Food Sci. Technol. 2017, 85, 500–505. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.A.; Bekheet, S.A. Mid-term Storage and Genetic Stability of Strawberry Tissue Cultures. Res. J. Agric. Biol. Sci. 2008, 4, 505–511. [Google Scholar]
- Shen, B.; Hohmann, S.; Jensen, R.G.; Bohnert, H.J. Roles of sugar alcohols in osmotic stress adaptation. Replacement of glycerol by mannitol and sorbitol in yeast. Plant Physiol. 1999, 121, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.-W.; Kwak, S.-H.; Yun, S.-Y.; Kim, S.-K. valuation of Antioxidant Activity of Sugar Alcohols Using TOSC (Total Oxy-radical Scavenging Capacity) Assay. Korean Soc. Toxicol. 2007, 23, 143–150. [Google Scholar] [CrossRef]
- Franco, M.J.; Martin, A.A.; Bonfim, L.F.; Caetano, J.; Linde, G.A.; Dragunski, D.C. Effect of Plasticizer and Modified Starch on Biodegradable Films for Strawberry Protection. J. Food Process. Preserv. 2017, 41, e13063. [Google Scholar] [CrossRef]
- Blanch, M.; Goñi, O.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Characterisation and functionality of fructo-oligosaccharides affecting water status of strawberry fruit (Fragraria vesca cv. Mara de Bois) during postharvest storage. Food Chem. 2012, 134, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Akšić, M.F.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-M.; van Eys, J. Nutritional Significance of Fructose and Sugar Alcohols. Annu. Rev. Nutr. 1981, 1, 437–475. [Google Scholar] [CrossRef]
- Dehghannya, J.; Hosseinlar, S.H.; Heshmati, K.M. Multi-stage continuous and intermittent microwave drying of quince fruit coupled with osmotic dehydration and low temperature hot air drying. Innov. Food Sci. Emerg. Technol. 2018, 45, 132–151. [Google Scholar] [CrossRef]
- Rasouli, M. Basic concepts and practical equations on osmolality: Biochemical approach. Clin. Biochem. 2016, 49, 936–941. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Association of Official Analytical Collaboration International. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Process. 2012, 90, 615–623. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The quality of red bell pepper subjected to freeze-drying preceded by traditional and novel pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Świeca, M. Hydrogen Peroxide Treatment and the Phenylpropanoid Pathway Precursors Feeding Improve Phenolics and Antioxidant Capacity of Quinoa Sprouts via an Induction of L-Tyrosine and L-Phenylalanine Ammonia-Lyases Activities. J. Chem. 2016, 2016, 1936516. [Google Scholar] [CrossRef] [Green Version]








| Sample | ΔMto (kg/kg) | ΔMtW (kg/kg) | ΔMtST (kg/kg) |
|---|---|---|---|
| Sucrose 50% | 0.603 ± 0.020 b | 0.657 ± 0.013 c | 0.054 ± 0.006 c |
| Mannitol 20% | 0.460 ± 0.037 a | 0.477 ± 0.031 a | 0.016 ± 0.006 ab |
| Mannitol 30% | 0.523 ± 0.023 ab | 0.564 ± 0.017 b | 0.041 ± 0.005 bc |
| Sorbitol 20% | 0.471 ± 0.008 a | 0.486 ± 0.007 a | 0.015 ± 0.001 a |
| Sorbitol 30% | 0.538 ± 0.020 ab | 0.578 ± 0.015 b | 0.041 ± 0.005 bc |
| Sorbitol 40% | 0.572 ± 0.032 b | 0.627 ± 0.023 bc | 0.055 ± 0.010 c |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Fresh | 34.3 ± 2.4 a | 25.8 ± 1.5 ab | 21.4 ± 3.4 a | - |
| Sucrose 50% | 43.6 ± 2.6 c | 26.3 ± 3.0 a | 17.9 ± 1.5 ab | 10.8 ± 2.1 ab |
| Mannitol 20% | 38.7 ± 1.7 b | 22.3 ± 2.5 ab | 12.9 ± 1.7 cd | 10.5 ± 2.1 ab |
| Mannitol 30% | 39.5 ± 2.9 b | 22.0 ± 2.6 b | 12.5 ± 2.6 d | 11.5 ± 2.5 b |
| Sorbitol 20% | 40.0 ± 2.7 bc | 24.0 ± 2.0 ab | 16.7 ± 2.3 bc | 8.3 ± 2.0 a |
| Sorbitol 30% | 40.9 ± 2.1 bc | 24.9 ± 2.5 ab | 16.3 ± 2.6 bcd | 8.9 ± 2.7 ab |
| Sorbitol 40% | 38.0 ± 1.3 ab | 26.3 ± 2.7 a | 15.0 ± 1.9 bcd | 7.9 ± 1.8 a |
| Osmotic Solution | Molar Mass of Substance (g/mol) | Osmolality 1 (mOsm/kg H2O) |
|---|---|---|
| sucrose 50% | 342.3 | 2921 |
| mannitol 20% | 182.2 | 1372 |
| mannitol 30% | 182.2 | 2353 |
| sorbitol 20% | 182.2 | 1372 |
| sorbitol 30% | 182.2 | 2353 |
| sorbitol 40% | 182.2 | 3660 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiktor, A.; Chadzynska, M.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules 2022, 27, 1376. https://doi.org/10.3390/molecules27041376
Wiktor A, Chadzynska M, Rybak K, Dadan M, Witrowa-Rajchert D, Nowacka M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules. 2022; 27(4):1376. https://doi.org/10.3390/molecules27041376
Chicago/Turabian StyleWiktor, Artur, Magdalena Chadzynska, Katarzyna Rybak, Magdalena Dadan, Dorota Witrowa-Rajchert, and Malgorzata Nowacka. 2022. "The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries" Molecules 27, no. 4: 1376. https://doi.org/10.3390/molecules27041376
APA StyleWiktor, A., Chadzynska, M., Rybak, K., Dadan, M., Witrowa-Rajchert, D., & Nowacka, M. (2022). The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules, 27(4), 1376. https://doi.org/10.3390/molecules27041376