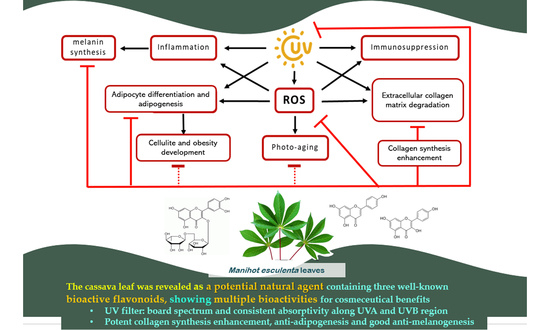
Multiple Bioactivities of Manihot esculenta Leaves: UV Filter, Anti-Oxidation, Anti-Melanogenesis, Collagen Synthesis Enhancement, and Anti-Adipogenesis
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition by HPLC-UV DAD Analysis
2.2. UV Absorption Covering UVA and UVB Rays
2.3. Anti-Oxidation
2.4. Anti-Melanogenesis in B16 Melanoma Cells
2.5. Collagen Synthesis Enhancement in NHDF Fibroblasts
2.6. Anti-Adipogenesis in 3T3-L1 Adipocytes
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Preparation of Cassava Leaf Extract
4.3. Analysis of Chemical Composition by High-Performance Liquid Chromatography
4.4. Determination of UV Screening Capacity
4.5. Anti-Oxidation by DPPH Radical Scavenging Assay
4.6. Anti-Melanogenesis in B16 Melanoma Cell Line
4.7. Collagen Synthesis Enhancement in NHDF Cell Line
4.8. Determination of Anti-Adipogenesis in 3T3-L1 Adipocytes
4.8.1. Preadipocyte Maintenance
4.8.2. Effect on Preadipocyte Viability Using MTT Assay
4.8.3. Inhibition of Preadipocyte Differentiation and Lipid Accumulation by Using Oil Red O Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miladiyah, I. Analgesic activity of ethanolic extract of Manihot esculenta Crantz leaves in mice. Universa Med. 2011, 30, 3–10. [Google Scholar]
- Linn, K.Z.; Myint, P.P. Estimation of nutritive value, total phenolic content and in vitro antioxidant activity of Manihot esculenta Crantz. (Cassava) leaf. J. Med. Plants 2018, 6, 73–78. [Google Scholar]
- Tao, H.; Cui, B.; Zhang, H.; Bekhit, A.E.-D.; Lu, F. Identification and characterization of flavonoids compounds in cassava leaves (Manihot esculenta Crantz) by HPLC/FTICR-MS. Int. J. Food Prop. 2019, 22, 1134–1145. [Google Scholar] [CrossRef] [Green Version]
- Tsumbu, C.N.; Deby-Dupont, G.; Tits, M.; Angenot, L.; Franck, T.; Serteyn, D.; Mouithys-Mickalad, A. Antioxidant and antiradical activities of Manihot esculenta Crantz (Euphorbiaceae) leaves and other selected tropical green vegetables investigated on lipoperoxidation and phorbol-12-myristate-13-acetate (PMA) activated monocytes. Nutrients 2011, 3, 818–838. [Google Scholar] [CrossRef] [Green Version]
- Moan, J. Visible light and UV radiation. In Radiation at Home, Outdoors and in the Workplace; Scandinavian Science Publisher: Oslo, Norway, 2001; pp. 69–85. [Google Scholar]
- Hsu, C.-L.; Yen, G.-C. Induction of cell apoptosis in 3T3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res. 2006, 50, 1072–1079. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, L.; Wei, H.; Chen, H.-D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. Adipocyte differentiation. In Adipose Tissue Biology; Springer: New York, NY, USA, 2017; pp. 69–90. [Google Scholar]
- Chaiittianan, R.; Chayopas, P.; Rattanathongkom, A.; Tippayawat, P.; Sutthanut, K. Anti-obesity potential of corn silks: Relationships of phytochemicals and anti-oxidation, anti-pre-adipocyte proliferation, anti-adipogenesis, and lipolysis induction. J. Funct. Foods 2016, 23, 497–510. [Google Scholar] [CrossRef]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal Extracts as Potential Antioxidant, Anti-Aging, Anti-Inflammatory, and Whitening Cosmeceutical Ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef]
- Rawtal, B.; Sahatpure, N.; Sakharwade, S. Pueraria tuberosa (vidarikanda): An emerging cosmeceutical herb. Int. J. Sci. 2019, 4, 130–137. [Google Scholar]
- Kamma, M.; Lin, W.-C.; Lau, S.-C.; Chansakaow, S.; Leelapornpisid, P. Anti-aging Cosmeceutical Product Containing of Nymphaea rubra Roxb. ex Andrews extract. Chiang Mai J. Sci. 2019, 46, 1143–1160. [Google Scholar]
- Hasnat, M.A.; Pervin, M.; Lim, B.O. Acetylcholinesterase Inhibition and in Vitro and in Vivo Antioxidant Activities of Ganoderma lucidum Grown on Germinated Brown Rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Venera, C. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Auh, J.-H.; Oh, J.; Hong, S.; Choi, S.; Shin, E.J.; Woo, S.O.; Lim, T.-G.; Byun, S. Propolis suppresses UV-induced photoaging in human skin through directly targeting phosphoinositide 3-kinase. Nutrients 2020, 12, 3790. [Google Scholar] [CrossRef]
- Ludriksone, L.; Tittelbach, J.; Schliemann, S.; Goetze, S.; Elsner, P. [When sunscreens do not help: Allergic contact dermatitis to UV filters]. Der Hautarzt Z. Fur Dermatol. Venerol. Und Verwandte Geb. 2018, 69, 941–944. [Google Scholar] [CrossRef]
- Siqueira, E.M.D.A.; Arruda, S.F.; de Vargas, R.M.; de Souza, E.M.T. β-Carotene from cassava (Manihot esculenta Crantz) leaves improves vitamin A status in rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 235–240. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Lulli, D.; Stancato, A.; De Luca, C.; Pastore, S.; Korkina, L. Modulation of Human Keratinocyte Responses to Solar UV by Plant Polyphenols As a Basis for Chemoprevention of Non-Melanoma Skin Cancers. Curr. Med. Chem. 2013, 20, 869–879. [Google Scholar]
- Degani, L.; Gulmini, M.; Piccablotto, G.; Iacomussi, P.; Gastaldi, D.; Dal Bello, F.; Chiantore, O. Stability of natural dyes under light emitting diode lamps. J. Cult. Herit. 2017, 26, 12–21. [Google Scholar] [CrossRef]
- Gers-Barlag, H.; Knueppel, A.; Mueller, A.; Staeb, F.; Doerschner, A.; Schoenrock, U. Compositions of Sulfonated UV Filter Materials with Flavone and/or Flavanone Derivatives, Especially Flavonoids, for Preparing Cosmetics. DE19923712. 2000. Available online: https://patents.google.com/patent/DE19923712A1/en (accessed on 11 November 2021).
- Pfluecker, F.; Buenger, J.; Driller, H.-J.; Buchholz, H.; Rosskopf, R. Flavonoid Compounds for Use against Oxidative Stress and UV Radiation. EP1205475. 2002. Available online: https://patents.google.com/patent/EP1205475B1/en (accessed on 8 November 2021).
- Endo, Y.; Usuki, R.; Kaneda, T. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. II. The mechanism of antioxidative action of chlorophyll. J. Am. Oil Chem. Soc. 1985, 62, 1387–1390. [Google Scholar] [CrossRef]
- Cai, W.; Chen, Y.; Xie, L.; Zhang, H.; Hou, C. Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur. Food Res. Technol. 2014, 238, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-N.; Kwon, Y.-I.; Jang, H.-D. Mulberry Leaf Extract Reduces Postprandial Hyperglycemia with Few Side Effects by Inhibiting α-Glucosidase in Normal Rats. J. Med. Food 2011, 14, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Kupeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Peres, D.D.A.; Ariede, M.B.; Candido, T.M.; de Almeida, T.S.; Lourenco, F.R.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Quality by design (QbD), Process Analytical Technology (PAT), and design of experiment applied to the development of multifunctional sunscreens. Drug Dev. Ind. Pharm. 2017, 43, 246–256. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, J.; Cheng, X.; Yi, B.; Zhang, X.; Li, Q. Apigenin induces dermalcollagen synthesis via smad2/3signaling pathway. Eur. J. Histochem. 2015, 59, 98–106. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Sun, Z.-w.; Lee, T.Y.; Song, H.G.; Shin, H.-S.; Yi, T.H. Vigna angularis Water Extracts Protect Against Ultraviolet B-Exposed Skin Aging In Vitro and In Vivo. J. Med. Food 2014, 17, 1339–1349. [Google Scholar] [CrossRef]
- Choi, B.-H.; Kim, S.-L.; Kim, S.-K. Rutin and functional ingredients of buckwheat and their variations. Korean J. Crop Sci. 1996, 41, 69–93. [Google Scholar]
- Si, Y.-X.; Yin, S.-J.; Oh, S.; Wang, Z.-J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An integrated study of tyrosinase inhibition by rutin: Progress using a computational simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, M.M.; Fontes, P.R.; Souza, P.M.; William Fagg, C.; Neves Silva Guerra, E.; de Medeiros Nóbrega, Y.K.; Silveira, D.; Fonseca-Bazzo, Y.; Simeoni, L.A.; Homem-de-Mello, M. Extracts of Morus nigra L. leaves standardized in chlorogenic acid, rutin and isoquercitrin: Tyrosinase inhibition and cytotoxicity. PLoS ONE 2016, 11, e0163130. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Wang, C.; Sun, B.; Qi, C. Understanding the role of extracts from sea buckthorn seed residues in anti-melanogenesis properties on B16F10 melanoma cells. Food Funct. 2018, 9, 5402–5416. [Google Scholar] [CrossRef] [PubMed]
- Juang, L.-J.; Gao, X.-Y.; Mai, S.-T.; Lee, C.-H.; Lee, M.-C.; Yao, C.-L. Safety assessment, biological effects, and mechanisms of Myrica rubra fruit extract for anti-melanogenesis, anti-oxidation, and free radical scavenging abilities on melanoma cells. J. Cosmet. Dermatol. 2019, 18, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Gosmann, G.; Barlette, A.G.; Dhamer, T.; Arcari, D.P.; Santos, J.C.; de Camargo, E.R.; Acedo, S.; Gambero, A.; Gnoatto, S.C.B.; Ribeiro, M.L. Phenolic Compounds from Mate (Ilex paraguariensis) Inhibit Adipogenesis in 3T3-L1 Preadipocytes. Plant Foods Hum. Nutr. 2012, 67, 156–161. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kee, J.-Y.; Park, J.; Kim, D.-S.; Shin, S.; Youn, D.-H.; Kang, J.; Jung, Y.; Lee, Y.-M.; Park, J.-H.; et al. Lipin1-Mediated Repression of Adipogenesis by Rutin. Am. J. Chin. Med. 2016, 44, 565–578. [Google Scholar] [CrossRef]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Migueliz, I.; Romo-Hualde, A.; Lopez-Yoldi, M.; Martinez, J.A.; Vizmanos, J.L.; Milagro, F.I.; Gonzalez-Navarro, C.J. Phenolic compounds inhibit 3T3-L1 adipogenesis depending on the stage of differentiation and their binding affinity to PPARγ. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Zorita, S.; Lasa, A.; Abendano, N.; Fernandez-Quintela, A.; Mosqueda-Solis, A.; Garcia-Sobreviela, M.P.; Arbones-Mainar, J.M.; Portillo, M.P. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-A.; Kang, K.; Lee, H.-J.; Kim, M.; Kim, C.Y.; Nho, C.W. Apigenin isolated from Daphne genkwa Siebold et Zucc. inhibits 3T3-L1 preadipocyte differentiation through a modulation of mitotic clonal expansion. Life Sci. 2014, 101, 64–72. [Google Scholar] [CrossRef]
- Su, T.; Huang, C.; Yang, C.; Jiang, T.; Su, J.; Chen, M.; Fatima, S.; Gong, R.; Hu, X.; Bian, Z.; et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol. Res. 2020, 152, 104586. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. The inhibitory effects of djulis (Chenopodium formosanum) and its bioactive compounds on adipogenesis in 3T3-L1 adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.S.; Wang, Z.; Lee, J.-M.; Lee, J.-Y.; Lim, S.S. Screening of Korean natural products for anti-adipogenesis properties and isolation of kaempferol-3-O-rutinoside as a potent anti-adipogenetic compound from Solidago virgaurea. Molecules 2016, 21, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, C.-C.; Ni, T.-W.; Yu, Y.; Qin, N.; Chen, Y.; Jin, M.-N.; Duan, H.-Q. Flavonoid derivative (Fla-CN) inhibited adipocyte differentiation via activating AMPK and up-regulating microRNA-27 in 3T3-L1 cells. Eur. J. Pharmacol. 2017, 797, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Phetpornpaisan, P.; Tippayawat, P.; Jay, M.; Sutthanut, K. A local Thai cultivar glutinous black rice bran: A source of functional compounds in immunomodulation, cell viability and collagen synthesis, and matrix metalloproteinase-2 and -9 inhibition. J. Funct. Foods 2014, 7, 650–661. [Google Scholar] [CrossRef]







| Chemical Constituent | Equation (y = ax) | Linear Regression (R2) | Content (mg/g Extract) |
|---|---|---|---|
| Gallic acid | y = 418656x | 0.9983 | 0.31 ± 0.00 |
| Rutin | y = 67947x | 0.9974 | 39.96 ± 0.81 |
| Kaempferol | y = 59296x | 0.9962 | 15.73 ± 0.19 |
| Apigenin | y = 118753x | 0.9981 | 27.70 ± 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampa, M.; Sutthanut, K.; Weerapreeyakul, N.; Tukummee, W.; Wattanathorn, J.; Muchimapura, S. Multiple Bioactivities of Manihot esculenta Leaves: UV Filter, Anti-Oxidation, Anti-Melanogenesis, Collagen Synthesis Enhancement, and Anti-Adipogenesis. Molecules 2022, 27, 1556. https://doi.org/10.3390/molecules27051556
Jampa M, Sutthanut K, Weerapreeyakul N, Tukummee W, Wattanathorn J, Muchimapura S. Multiple Bioactivities of Manihot esculenta Leaves: UV Filter, Anti-Oxidation, Anti-Melanogenesis, Collagen Synthesis Enhancement, and Anti-Adipogenesis. Molecules. 2022; 27(5):1556. https://doi.org/10.3390/molecules27051556
Chicago/Turabian StyleJampa, Manuschanok, Khaetthareeya Sutthanut, Natthida Weerapreeyakul, Wipawee Tukummee, Jintanaporn Wattanathorn, and Suparporn Muchimapura. 2022. "Multiple Bioactivities of Manihot esculenta Leaves: UV Filter, Anti-Oxidation, Anti-Melanogenesis, Collagen Synthesis Enhancement, and Anti-Adipogenesis" Molecules 27, no. 5: 1556. https://doi.org/10.3390/molecules27051556
APA StyleJampa, M., Sutthanut, K., Weerapreeyakul, N., Tukummee, W., Wattanathorn, J., & Muchimapura, S. (2022). Multiple Bioactivities of Manihot esculenta Leaves: UV Filter, Anti-Oxidation, Anti-Melanogenesis, Collagen Synthesis Enhancement, and Anti-Adipogenesis. Molecules, 27(5), 1556. https://doi.org/10.3390/molecules27051556