Hydrogenation of β-Keto Sulfones to β-Hydroxy Sulfones with Alkyl Aluminum Compounds: Structure of Intermediate Hydroalumination Products
Abstract
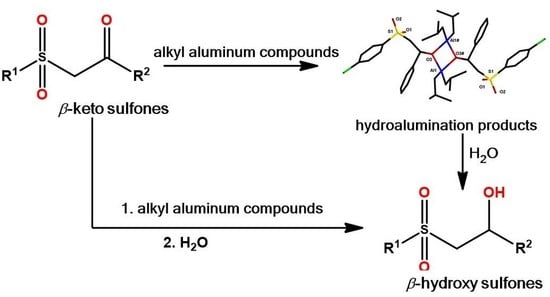
:1. Introduction
2. Results and Discussion
2.1. Hydroalumination Reaction of β-Keto Sulfones
2.2. Hydrogenation of β-Keto Sulfones to β-Hydroxy Sulfones
3. Materials and Methods
3.1. General Remarks
3.2. X-ray Crystallography
3.3. Reactions of β-Keto Sulfones with Alkyl Aluminum Compounds—General Procedure
3.4. Preparation of Hydroalumination Products
Reactions of i-Bu3Al, i-Bu2AlH and Et3Al with β-Keto Sulfones
3.5. Preparation of β-Hydroxy Sulfones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achmatowicz, B.; Baranowska, E.; Daniewski, A.R.; Pankowski, J.; Wicha, J. BF3-mediated reaction of a sulfone with aldehydes. A method for stereospecific construction of prostaglandin ω-chain. Tetrahedron 1988, 44, 4989–4998. [Google Scholar] [CrossRef]
- Kocienski, P.J.; Lythgoe, B.; Ruston, S. Scope and stereochemistry of an olefin synthesis from β-hydroxy-sulphones. J. Chem. Soc. Perkin Trans. 1 1978, 8, 829–834. [Google Scholar] [CrossRef]
- Kocienski, P.J.; Lythgoc, B.; Waterhouse, I. The influence of chain-branching on the steric outcome of some olefin forming Reactions. J. Chem. Soc. Perkin Trans. 1 1980, 1045–1050. [Google Scholar] [CrossRef]
- Kocienski, P.J. A Synthesis of Moenocinol. J. Org. Chem. 1980, 45, 2037–2039. [Google Scholar] [CrossRef]
- Julia, M.; Paris, J.M. Syntheses a l’aide de sufones V(+)—Method de synthese generale de doubles liaisons. Tetrahedron Lett. 1973, 14, 4833–4836. [Google Scholar] [CrossRef]
- Otera, J.; Misawa, H.; Sugimoto, K.J. Mechanistic aspects and profiles of the double elimination reaction of β-substituted sulfones. Org. Chem. 1986, 51, 3830–3833. [Google Scholar] [CrossRef]
- Solladie, G.; Frechou, G.; Demailly, G.; Greek, C. Reduction of chiral β-hydroxy sulfoxides: Application to the synthesis of both enantiomers of 4-substituted butenolides. J. Org. Chem. 1986, 51, 1912–1914. [Google Scholar] [CrossRef]
- Chan, C.-K.; Chen, Y.-H.; Hsu, R.-T.; Chang, M.-Y. Synthesis of γ-sulfonyl δ-lactones and β-sulfonyl styrenes. Tetrahedron 2017, 73, 46–54. [Google Scholar] [CrossRef]
- Tanikaga, R.; Hosoya, K.; Kaji, A. Synthesis of enantiomerically pure 2,5-disubstituted tetrahydrofurans using readily prepared (2S)-1-phenylsulphonylalkan-2-ols. J. Chem. Soc. Perkin Trans. 1 1987, 1799–1803. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Lu, Y.-J.; Cheng, Y.-C. m-CPBA-mediated stereoselective synthesis of sulfonyl tetrahydropyrans. Tetrahedron 2015, 71, 1192–1201. [Google Scholar] [CrossRef]
- Bertus, P.; Phansavath, P.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Touati, A.; Homri, T.; Hassine, B.B. Enantioselective hydrogenation of β-keto sulfones with chiral Ru(II)-catalysts: Synthesis of enantiomerically pure butenolides and γ-butyrolactones. Tetrahedron Asymm. 1999, 10, 1369–1380. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Mugrage, B.B.; Li, C.S.; Felder, L. Chemistry of baker’s yeast reduction products: Use of optically active (S)-(+)-1-(p-toluenesulfonyl)propan-2-ol and (S)-(+)-1-(phenylsulfonyl)propan-2-ol in synthesis. Tetrahedron Lett. 1986, 27, 4817–4820. [Google Scholar] [CrossRef]
- Tanikaga, R.; Hosoya, K.; Kaji, A. Reactions of (2S)-1-arenesulfonyl-2-alkanol dianions with aldehydes, application to the synthesis of enantiomerically pure (3S)-1-alken-3-ols and (2E,4S)-4-hydroxy-2-alkenenitriles. Chem. Lett. 1987, 16, 829–832. [Google Scholar] [CrossRef] [Green Version]
- Najera, C.; Sansano, J.M. Synthesis of β- and γ-hydroxy sulfones by regioselective opening of β,γ-epoxy sulfones. Tetrahedron 1990, 46, 3993–4002. [Google Scholar] [CrossRef]
- Maiti, A.K.; Bhattacharyya, P. Polyethylene Glycol (PEG) 4000 Catalyzed regioselective nucleophilic ring opening of oxiranes—A new and convenient Synthesis of β-hydroxy sulfone and β-hydroxy sulfide. Tetrahedron 1994, 50, 10483–10490. [Google Scholar] [CrossRef]
- Narayana Murthy, S.; Madhav, B.; Prakash Reddy, V.; Rama Rao, K.; Nageswar, Y.V.D. An approach toward the synthesis of β-hydroxy sulfones on water. Tetrahedron Lett. 2009, 50, 5009–5011. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Kuo, Y.-C.; Kuei, C.-H.; Chang, M.-Y. Palladium-mediated synthesis of 1,1,2-triarylethanes. Application to the synthesis of CDP-840. Tetrahedron 2017, 73, 1275–1282. [Google Scholar] [CrossRef]
- Chang, C.; Cheng, Y.-C. Stereocontrolled synthesis of sulfonyl 2,5-diaryltetrahydrofurans. Synlett 2016, 27, 854–858. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Huang, Y.-H.; Wang, H.-S. Synthesis of oxygenated 1-arylnaphthalenes. Tetrahedron 2016, 72, 1888–1895. [Google Scholar] [CrossRef]
- Muneeswara, M.; Sundaravelu, N.; Sekar, G. NBS-mediated synthesis of β-keto sulfones from benzyl alcohols and sodium arenesulfinates. Tetrahedron 2019, 75, 3479–3484. [Google Scholar] [CrossRef]
- Tao, L.; Yin, C.; Dong, X.-Q.; Zhang, X. Org. Efficient synthesis of chiral β-hydroxy sulfones via iridium-catalyzed hydrogenation. Biomol. Chem. 2019, 17, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Zhang, S.-Y.; Geng, Z.-C.; Kwok, C.-Y.; Liu, P.; Li, H.-Y.; Wang, X.-W. Asymmetric hydrogenation of β-keto sulfonamides and β-keto sulfones with a chiral cationic ruthenium diamine catalyst. Adv. Synth. Catal. 2013, 355, 2860–2872. [Google Scholar] [CrossRef]
- Cui, P.; Liu, Q.; Wang, J.; Liu, H.; Zhou, H. One-pot synthesis of chiral β-hydroxysulfones from alkynes via aerobic oxysulfonylation and asymmetric reduction in MeOH/H2O. Green Chem. 2019, 21, 634–639. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Hou, X.-L.; Dai, L.-X.; Luo, Z.-B. Synthesis of a biferrocene diphosphine ligand with only planar chirality and its application in the Rh-catalyzed asymmetric hydrogenation of β-keto sulfones. Tetrahedron Asymm. 2007, 18, 224–228. [Google Scholar] [CrossRef]
- Wan, X.; Meng, Q.; Zhang, H.; Sun, Y.; Fan, W.; Zhang, Z. An efficient synthesis of chiral β-hydroxy sulfones via Ru-catalyzed enantioselective hydrogenation in the presence of iodine. Org. Lett. 2007, 9, 5613–5616. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, T.; Ochal, Z.; Socha, P.; Dobrzycki, Ł.; Ziemkowska, W. Reactions of β-keto sulfones with t-butyl aluminum compounds: Reinvestigation of tri-t-butyl aluminum synthesis. Appl. Organomet. Chem. 2020, 34, e5961. [Google Scholar] [CrossRef]
- Ashby, E.C.; Yu, S.H. Organometallic reaction mechanisms. IV. Mechanism of ketone reduction by aluminum alkyls. J. Org. Chem. 1970, 35, 1034–1040. [Google Scholar] [CrossRef]
- Bundens, J.W.; Seida, P.R.; Jeyakumar, D.; Francl, M.M. An ab initio molecular orbital study of the reduction of carbonyls by alkylaluminum complexes. J. Mol. Graph. Model. 2005, 24, 195–202. [Google Scholar] [CrossRef]
- Eisch, J.J.; Fichter, K.C. Organometallic compounds of group 13. XXXI. Stereochemistry of ketone insertion and enol salt formation at alkyl carbon-aluminum bonds. J. Am. Chem. Soc. 1975, 97, 4772–4774. [Google Scholar] [CrossRef]
- Giacomelli, G.P.; Menicagli, R.; Lardicci, L. Alkyl metal asymmetric reduction. 7. Temperature-dependence of stereosectivity of alkyl phenyl ketone reductions by chiral organoaluminum compounds. J. Am. Chem. Soc. 1975, 97, 4009–4012. [Google Scholar] [CrossRef]
- Giacomelli, G.P.; Menicagli, R.; Lardicci, L. Alkyl metal asymmetric reduction. Stereochemistry of alkyl phenyl ketone reductions by chiral organoaluminum compounds. J. Org. Chem. 1973, 38, 2370–2376. [Google Scholar] [CrossRef]
- Heinsohn, G.E.; Ashby, E.C. Stereochemistry of reduction of substituted cyclohexanones with triisobutylaluminum and diisobutylaluminum hydride. J. Org. Chem. 1973, 38, 4232–4236. [Google Scholar] [CrossRef]
- Ashby, E.C.; Laemmle, J.T. Stereoselective organometallic alkylation reactions. 4. Organolithium and organoaluminum addition to trimethylaluminum, triphenylaluminum and trichloroaluminum complexes of 4-tert-butylcyclohexanone and 2-methylcyclopentanone. J. Org. Chem. 1975, 40, 1469–1475. [Google Scholar] [CrossRef]
- Giacomelli, G.; Caporusso, A.M.; Lardicci, L. Alkyl metal asymmetric reduction. 11. The reaction of alpha, beta-unsaturated ketones with beta-branched trialkylaluminum compounds. Tetrahedron Lett. 1981, 22, 3663–3666. [Google Scholar] [CrossRef]
- Eisch, J.J.; Foxton, M.W. Organometallic compounds of Group III. XIX. Regiospecificity and stereochemistry in the hydralumination of unsymmetrical acetylenes. Controlled cis or trans reduction of 1-alkynyl derivatives. J. Org. Chem. 1971, 36, 3520–3526. [Google Scholar] [CrossRef]
- Eisch, J.J.; Gopal, H.; Rhee, S.-G. Organometallic compounds of Group III. Regiochemistry and stereochemistry in the hydralumination of heterosubstituted acetylenes. Interplay of inductive and resonance effects in electron-rich alkynes. J. Org. Chem. 1975, 40, 2064–2069. [Google Scholar] [CrossRef]
- Uhl, W. Hydroalumination and hydrogallation of alkynes: New insights into the course of well-known reactions. Coord. Chem. Rev. 2008, 252, 1540–1563. [Google Scholar] [CrossRef]
- Lee, B.; Shin, M.; Seo, Y.; Kim, H.M.; Lee, R.H.; Kim, S.J.; Chung, K.; Yoo, D.; Kim, G.Y. Synthesis of 2,4,6,8,9,11-hexaaza[3.3.3]propellanes as a new molecular skeleton for explosives. Tetrahedron 2018, 74, 130–134. [Google Scholar] [CrossRef]
- Konysheva, A.V.; Tolmacheva, I.A.; Savinova, O.V.; Boreko, E.I.; Grishko, V.V. Regioselective transformation of the cyano group of triterpene α,β-alkenenitriles. Chem. Nat. Comp. 2017, 53, 687–690. [Google Scholar] [CrossRef]
- Ducry, L.; Roberge, M.D. Dibal-H reduction of methyl butyrate into butyraldehyde using microreactors. Org. Process Res. Dev. 2008, 12, 163–167. [Google Scholar] [CrossRef]
- Sierra, M.L.; Kumar, R.; de Mel, V.S.J.; Oliver, J.P. Synthesis and spectroscopic investigations of alkylaluminum alkoxides derived from optically active alcohols. The first structural identification of an optically active organoaluminum alkoxide. Organometallics 1992, 11, 206–214. [Google Scholar] [CrossRef]
- Sierra, M.L.; Kumar, R.; de Mel, V.S.J.; Oliver, J.P. Synthesis and spectroscopic investigations of alkylaluminum derivatives of 2-allyl-6-methylphenoxide and 2-naphthoxide: Crystal structure of [Me2Al(.mu.-2-allyl-6-methylphenoxide)]2. Organometallics 1990, 9, 484–489. [Google Scholar] [CrossRef]
- Basiak, D.; Ochal, Z.; Justyniak, I.; Ziemkowska, W. 1-(1,3-Benzothiazol-2-ylsulfanyl)propan-2-olate anion as a potential multifunctional ligand in aluminum complexes. Polyhedron 2015, 102, 705–710. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Abdel-Fattah, A.A.A.; Wang, M. Efficient conversion of sulfones into β-keto sulfones by N-acylbenzotriazoles. J. Org. Chem. 2003, 68, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX2; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Bruker. Data Reduction Software, SAINT; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- SADABSe2012/1 Bruker/Siemens. Area Detector Absorption Correction Program; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Cryst. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.J.C.; Geist, V. Mathematical, Physical and Chemical Tables. In International Tables for Crystallography; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; Volume C. [Google Scholar] [CrossRef]
- Chan, C.-K.; Lo, N.-C.; Chen, P.-Y.; Chang, M.-Y. An efficient organic electrosynthesis of β-hydroxysulfones. Synthesis 2017, 49, 4469–4477. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, W.; Huo, C. One-pot synthesis of β-hydroxysulfones and its application in the preparation of anticancer drug bicalutamide. J. Org. Chem. 2017, 82, 10628–10634. [Google Scholar] [CrossRef]
- Son, S.; Shyam, P.K.; Park, H.; Jeong, I.; Jang, H.-Y. Complementary strategy for regioselective synthesis of diverse β-hydroxysulfones from thiosulfonates. Eur. J. Org. Chem. 2018, 83, 3365–3371. [Google Scholar] [CrossRef]
- Taniguchi, N. Aerobic nickel-catalyzed hydroxysulfonylation of alkenes using sodium sulfinates. J. Org. Chem. 2015, 80, 7797–7802. [Google Scholar] [CrossRef]
- Chumachenko, N.; Sampson, P. Synthesis of β-hydroxy sulfones via opening of hydrophilic epoxides with zinc sulfinates in aqueous media. Tetrahedron 2006, 62, 4540–4548. [Google Scholar] [CrossRef]
- Fiandanese, V.; Maffeo, C.V.; Naso, F.; Ronzini, L. Mechanistic study of syn- and anti-elimination from diastereoisomeric halogenosulphonylethanes. J. Chem. Soc. Perkin Trans. 1976, 2, 1303–1307. [Google Scholar] [CrossRef]
- Field, L.; McFarland, J.W. Grignard Reagents of Sulfones. II. Reactions with Carbonyl Compounds. J. Am. Chem. Soc. 1953, 75, 5582–5586. [Google Scholar] [CrossRef]
- Truce, W.E.; Klingler, T.C. Synthesis and configurational assignments of diastereomeric beta-hydroxy sulfones. J. Org. Chem. 1970, 35, 1834–1838. [Google Scholar] [CrossRef]









| Run | β-Keto Sulfone | Alkyl Aluminum Reagents | Molar Ratio a | Solvent | Yield Molar Ratio b | β-Hydroxy Sulfone |
|---|---|---|---|---|---|---|
| 1. |  1a | i-Bu3Al c i-Bu2AlH c Et3Al c i-Bu3Al i-Bu2AlH Et3Al Et3Al n-Bu3Al n-Bu3Al n-Hex3Al n-Hex3Al EtAlCl2 EtAlCl2 | 1:1 1:1 1:1 1:1 1:1 1:1 1:2 1:1 1:1 1:1 1:3 1:1 1:2 | CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 C6H5CH3 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 | 100:0 c 100:0 c 100:0 c 100:0 100:0 100:0 100:0 76:24 52:48 55:45 100:0 0:100 0:100 |  4a |
| 2. |  1b | i-Bu3Al c i-Bu2AlH c Et3Al c Et3Al c i-Bu3Al i-Bu2AlH Et3Al Et3Al t-Bu3Al t-Bu3Al Et2AlCl Et2AlCl | 1:1 1:1 1:2 1:1 1:1 1:1 1:2 1:1 1:1 1:2 1:1 1:2 | CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 CH2Cl2 n-C5H12 n-C5H12 CH2Cl2 CH2Cl2 | 100:0 c 100:0 c 100:0 c 100:0 c 100:0 100:0 100:0 100:0 8:92 92:8 17:83 25:75 |  4b |
| 3. |  1c | Et3Al Et3Al i-Bu3Al | 1:1 1:2 1:1 | CH2Cl2 CH2Cl2 CH2Cl2 | 75:25 82:18 100:0 |  4c |
| 4. |  1d | Et3Al Et3Al i-Bu3Al | 1:1 1:2 1:1 | CH2Cl2 CH2Cl2 CH2Cl2 | 100:0 100:0 100:0 |  4d |
| 5. |  1e | Et3Al Et3Al i-Bu3Al | 1:1 1:2 1:1 | CH2Cl2 CH2Cl2 CH2Cl2 | 100:0 100:0 100:0 |  4e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotecki, M.; Ochal, Z.; Socha, P.; Szejko, V.; Dobrzycki, Ł.; Stypik, M.; Ziemkowska, W. Hydrogenation of β-Keto Sulfones to β-Hydroxy Sulfones with Alkyl Aluminum Compounds: Structure of Intermediate Hydroalumination Products. Molecules 2022, 27, 2357. https://doi.org/10.3390/molecules27072357
Kotecki M, Ochal Z, Socha P, Szejko V, Dobrzycki Ł, Stypik M, Ziemkowska W. Hydrogenation of β-Keto Sulfones to β-Hydroxy Sulfones with Alkyl Aluminum Compounds: Structure of Intermediate Hydroalumination Products. Molecules. 2022; 27(7):2357. https://doi.org/10.3390/molecules27072357
Chicago/Turabian StyleKotecki, Michał, Zbigniew Ochal, Paweł Socha, Vadim Szejko, Łukasz Dobrzycki, Mariola Stypik, and Wanda Ziemkowska. 2022. "Hydrogenation of β-Keto Sulfones to β-Hydroxy Sulfones with Alkyl Aluminum Compounds: Structure of Intermediate Hydroalumination Products" Molecules 27, no. 7: 2357. https://doi.org/10.3390/molecules27072357
APA StyleKotecki, M., Ochal, Z., Socha, P., Szejko, V., Dobrzycki, Ł., Stypik, M., & Ziemkowska, W. (2022). Hydrogenation of β-Keto Sulfones to β-Hydroxy Sulfones with Alkyl Aluminum Compounds: Structure of Intermediate Hydroalumination Products. Molecules, 27(7), 2357. https://doi.org/10.3390/molecules27072357