Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods
Abstract
:1. Introduction
2. Results and Discussions
2.1. Total Content of Phenolic Compounds, Flavonoids, and Radical Scavenging Activity
2.2. Qualitative Composition of Volatile Compounds
2.3. Xanthohumol Content and Variation of Bitter Acids
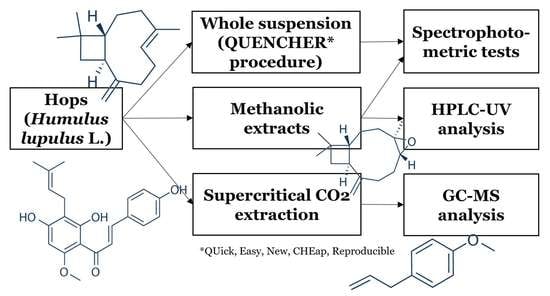
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Assessment of Sample Moisture
3.4. Preparation of Solvent Extracts
3.5. Spectrophotometric Analysis of Liquid Extracts
3.6. QUENCHER Procedure of the Suspended Material
3.7. Preparation of Supercritical CO2 Extracts
3.8. Gas Chromatography-Mass Spectrometry
3.9. High-Performance Liquid Chromatography
3.10. Statistical Analysis
4. Conclusions
- (a)
- The QUENCHER procedure shows 1.2–3.5 times more total content of phenolic compounds than the classical method with conventional solvent extraction.
- (b)
- The QUENCHER procedure reveals matrix encapsulated/adsorbed/insoluble substances.
- (c)
- The leaves of the hops contain phenolic compounds that are insoluble in aqueous 75% methanol.
- (d)
- The content of phenolic compounds and antioxidant activity correlates.
- (e)
- The highest value of phenolic compounds and radical scavenging activity was in the suspensions of leaves and cones of Fredos taurieji’ and ‘Fredos derlingieji’.
- (f)
- The highest concentration of xanthohumol in the leaves was 0.0080%, and in the cones was 0.2136% of dry mass.
- (g)
- The xanthohumol content in hop cones strongly correlates with flavonoid, bitter α-acids, and β-acids contents.
- (h)
- The highest amounts of hop’s bitter acids were determined in ‘Kauno gražieji’ and ‘Raudoniai’.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef] [PubMed]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Mǒzina, S.S.; Kǒsir, I.J.; Kǎc, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crops Prod. 2015, 67, 124–134. [Google Scholar] [CrossRef]
- Aberl, A.; Coelhan, M. Determination of volatile compounds in different hop varieties by headspace-trap GC/MS—In comparison with conventional hop essential oil analysis. J. Agric. Food Chem. 2012, 60, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Krofta, K.; Mikyška, A.; Hašková, D. Antioxidant characteristics of hops and hop products. J. Inst. Brew. 2008, 114, 160–166. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Gill, C.; Rowland, I. A comparison of the anti-cancer properties of isoxanthohumol and 8-prenylnaringenin using in vitro models of colon cancer. BioFactors 2013, 39, 441–447. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef]
- Sansawat, T.; Lee, H.C.; Zhang, L.; Ryser, E.; Kang, I. Antilisterial effects of different hop acids in combination with potassium acetate and potassium diacetate at 7 and 37 °C. Food Control 2016, 59, 256–261. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, S.; Xu, L.; Lu, Y.; Lu, Z.; Chen, C.; Ni, J.; Wan, R.; Yang, L. The inhibitory effects of xanthohumol, a prenylated chalcone derived from hops, on cell growth and tumorigenesis in human pancreatic cancer. Biomed. Pharmacother. 2015, 73, 40–47. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Król, S.K.; Dmoszyńska-Graniczka, M.; Zdzisińska, B.; Stepulak, A.; Gagos, M. Xanthohumol inhibits cell cycle progression and proliferation of larynx cancer cells in vitro. Chem. Biol. Interact. 2015, 240, 110–118. [Google Scholar] [CrossRef]
- Jiang, C.H.; Sun, T.L.; Xiang, D.X.; Wei, S.S.; Li, W.Q. Anti-cancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Z.H.; Liu, J.K.; Zheng, Y.T. Xanthohumol, a novel anti-HIV-1 agent purified from hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Stanius, Ž.; Obelevičius, K.; Kornyšova, O.; Maruška, A.; Ragažinskienė, O. Capillary electrophoresis method for determination of bitter ( α- and β- ) acids in Humulus lupulus L. Lithuanian varieties. Biologija 2005, 3, 81–84. [Google Scholar]
- Kac, J.; Vovk, T. Sensitive electrochemical detection method for α-acids, β-acids and xanthohumol in hops (Humulus lupulus L.). J. Chromatogr. B 2007, 850, 531–537. [Google Scholar] [CrossRef]
- Ceh, B.; Kac, M.; Košir, I.; Abram, V. Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef] [Green Version]
- Ligor, M.; Stankevičius, M.; Wenda-Piesik, A.; Obelevičius, K.; Ragažinskienė, O.; Stanius, Ž.; Maruška, A.; Buszewski, B. Comparative gas chromatographic–mass spectrometric evaluation of hop (Humulus lupulus L.) essential oils and extracts obtained using different sample preparation methods. Food Anal. Methods 2014, 7, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Horák, T.; Čulík, J.; Čejka, P.; Jurková, M.; Kellner, V.; Dvořák, J.; Hašková, D. Analysis of free fatty acids in beer: Comparison of solid-phase extraction, solid-phase microextraction, and stir bar sorptive extraction. J. Agric. Food Chem. 2009, 57, 11081–11085. [Google Scholar] [CrossRef]
- Kovačevič, M.; Kač, M. Solid-phase microextraction of hop volatiles. J. Chromatogr. A 2001, 918, 159–167. [Google Scholar] [CrossRef]
- Dzingelevičius, N.; Maruška, A.; Ragažinskienė, O.; Obelevičius, K. Optimization of hop essential oil extraction by means of supercritical CO2. Biologija 2011, 57, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Kaškonas, P.; Stanius, Z.; Kaškonienė, V.; Obelevičius, K.; Ragažinskienė, O.; Žilinskas, A.; Maruška, A. Clustering analysis of different hop varieties according to their essential oil composition measured by GC/MS. Chem. Pap. 2016, 70, 1568–1577. [Google Scholar] [CrossRef]
- Henrion, M.; Servaes, M.; Thielecke, F.; Fogliano, V. Application of the QUENCHER methodology to the food industry. Food Chem. 2018, 240, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Kaškonas, P.; Jalinskaitė, M.; Maruška, A. Chemical composition and chemometric analysis of variation in essential oils of Calendula officinalis L. during vegetation stages. Chromatographia 2011, 73, 163–169. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A.; Ragažinskienė, O. Essential oils of Bidens tripartita L. collected during period of 3 years composition variation analysis. Acta Physiol. Plant. 2013, 35, 1171–1178. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Palombini, S.V.; Maruyama, S.A.; Claus, T.; Carbonera, F.; De Souza, N.E.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Evaluation of antioxidant potential of Brazilian rice cultivars. Food Sci. Technol. Camp. 2013, 33, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Hart, J.M.; Christensen, N.W. Nitrogen Uptake and Utilization by Pacific Northwest Crops. PNW 513; A Pacific Northwest Publication; Oregon State University Extension Service: Corvallis, OR, USA, 1999. [Google Scholar]
- Kaškonienė, V.; Ruočkuvienė, G.; Kaškonas, P.; Akuneca, I.; Maruška, A. Chemometric analysis of bee pollen based on volatile and phenolic compound compositions and antioxidant properties. Food Anal. Methods 2015, 8, 1150–1163. [Google Scholar] [CrossRef]



| Variety | Total Phenolic Compounds (mg RE/g) | Total Flavonoids (mg RE/g) | Radical Scavenging Activity (mg RE/g) | |||
|---|---|---|---|---|---|---|
| Extract | QUENCHER | Extract | QUENCHER | Extract | QUENCHER | |
| Leaves | ||||||
| RA | 53.00 ± 0.44 a | 128.35 ± 3.14 e | 15.92 ± 0.61 a | 21.41 ± 1.97 f | 39.77 ± 0.69 a | 52.69 ± 5.38 d |
| FT | 60.46 ± 2.43 b | 168.46 ± 9.44 f | 18.08 ± 0.40 b | 18.76 ± 1.08 bcf | 67.83 ± 0.78 b | 121.02 ± 12.09 e |
| FD | 63.59 ± 1.59 b | 164.18 ± 15.53 f | 16.38 ± 0.28 ac | 14.76 ± 1.8 adg | 69.51 ± 1.13 b | 108.71 ± 5.49 e |
| KA | 39.19 ± 0.14 c | 127.25 ± 6.00 e | 13.14 ± 0.12 d | 14.45 ± 0.97 adg | 34.40 ± 0.56 c | 51.93 ± 4.58 d |
| KG | 31.40 ± 1.25 d | 111.40 ± 5.00 g | 11.00 ± 0.34 e | 12.95 ± 1.09 dg | 34.56 ± 0.73 c | 48.43 ± 4.82 d |
| Cones | ||||||
| RA | 71.72 ± 1.31 a | 102.28 ± 8.60 e | 18.64 ± 0.40 a | 17.88 ± 1.87 abcf | 65.14 ± 0.78 a | 92.82 ± 4.8 bf |
| FT | 78.18 ± 0.65 b | 122.11 ± 5.50 f | 17.87 ± 0.38 b | 16.50 ± 1.62 acdfg | 87.16 ± 0.82 b | 101.97 ± 10.02 f |
| FD | 72.39 ± 0.30 c | 134.77 ± 12.87 f | 19.19 ± 0.46 ac | 12.65 ± 2.21 dg | 74.29 ± 0.79 c | 95.68 ± 9.27 bdf |
| KA | 76.91 ± 2.43 d | 91.74 ± 8.71 e | 14.45 ± 0.43 d | 12.66 ± 1.68 cdg | 82.42 ± 1.28 d | 69.34 ± 5.14 aceg |
| KG | 76.73 ± 1.67 d | 120.82 ± 7.69 ef | 23.31 ± 0.58 e | 20.18 ± 0.61 cf | 70.80 ± 0.93 e | 66.63 ± 5.53 aceg |
| Rt, min | Identified Compound | KI | Leaves | Cones | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RA | FT | FD | KA | KG | RA | FT | FD | KA | KG | |||
| 7.277 | β-Pinene | 983 | 0.41 | 0.43 | 0.51 | 0.52 | 0.42 | |||||
| 7.853 | β-Myrcene | 995 | 26.88 | 26.49 | 23.42 | 32.62 | 34.59 | |||||
| 9.439 | Sylvestrene | 1028 | 0.17 | 0.16 | 0.20 | 0.26 | 0.32 | |||||
| 16.737 | Borneol | 1166 | 0.52 | 3.55 | 1.91 | 1.76 | 1.68 | |||||
| 18.898 | Estragole | 1203 | 1.82 | 12.17 | 5.20 | 7.31 | 17.94 | 0.12 | ||||
| 29.074 | Ylangene | 1368 | 0.08 | 0.10 | 0.10 | 0.06 | 0.06 | |||||
| 29.349 | Copaene | 1373 | 0.89 | 0.94 | 1.22 | 0.32 | 0.43 | 0.42 | 0.22 | 0.22 | ||
| 31.923 | β-Caryophyllene | 1415 | 2.48 | 13.92 | 24.81 | 16.85 | 13.59 | 9.65 | 12.14 | 12.56 | 7.62 | 7.84 |
| 32.550 | β-Cubebene | 1426 | 0.94 | 0.42 | 0.54 | 0.53 | 0.33 | 0.36 | ||||
| 33.111 | α-Bergamotene | 1435 | 0.93 | 0.72 | 0.70 | 0.88 | 0.85 | |||||
| 33.984 | α-Humulene | 1449 | 8.55 | 38.01 | 50.56 | 58.55 | 50.06 | 34.49 | 37.09 | 37.31 | 27.59 | 27.79 |
| 34.586 | β-Farnesene | 1460 | 0.96 | 3.01 | 4.17 | 3.77 | 5.02 | 21.24 | 15.21 | 14.59 | 23.21 | 20.57 |
| 35.279 | β-Cadinene | 1471 | 0.05 | 0.22 | 0.22 | 0.15 | 0.12 | |||||
| 35.496 | γ-Muurolene | 1475 | 0.23 | 1.43 | 2.66 | 1.50 | 1.99 | 0.60 | 0.90 | 0.91 | 0.63 | 0.64 |
| 35.936 | β-Selinene | 1482 | 0.24 | 0.34 | 0.37 | 0.39 | 0.35 | |||||
| 36.541 | γ-Amorphene | 1492 | 0.79 | 0.36 | 0.55 | 0.60 | 0.43 | 0.36 | ||||
| 36.903 | α-Farnesene | 1498 | 0.53 | 0.88 | 1.06 | 0.86 | 0.26 | |||||
| 37.662 | γ-Cadinene | 1512 | 0.74 | 1.67 | 2.15 | 2.97 | 2.53 | 1.11 | 1.29 | 1.42 | 1.25 | 0.92 |
| 38.292 | δ-Cadinene | 1523 | 1.01 | 2.91 | 3.77 | 4.77 | 3.25 | 1.41 | 1.73 | 1.99 | 1.33 | 1.19 |
| 38.722 | trans-Cadina-1,4-diene | 1530 | 0.13 | 0.13 | 0.18 | 0.15 | 0.11 | |||||
| 39.030 | α-Muurolene | 1536 | 0.11 | 0.13 | 0.17 | 0.08 | 0.11 | |||||
| 41.494 | Caryophyllene oxide | 1579 | 0.15 | 0.05 | 0.07 | 0.10 | 0.23 | |||||
| 42.996 | Humulene epoxide II | 1606 | 1.30 | 3.14 | 0.44 | 0.19 | 0.22 | 0.41 | 0.87 | |||
| - | Sum of minor compounds a | 83.69 | 22.44 | 2.10 | 0.00 | 0.80 | 0.16 | 0.28 | 2.45 | 0.91 | 1.82 | |
| Variety | Xanthohumol Content (% of Dry Weight) | |
|---|---|---|
| Leaves | Cones | |
| ‘Raudoniai’ | 0.0014 a | 0.1466 f |
| ‘Fredos taurieji’ | 0.0080 b | 0.1181 g |
| ‘Fredos derlingieji’ | 0.0067 c | 0.1295 h |
| ‘Kauno ankstyvieji’ | 0.0059 d | 0.1413 f |
| ‘Kauno gražieji’ | 0.0030 e | 0.2136 i |
| Variety | Sample Code | Country of Origin | Voucher No. |
|---|---|---|---|
| ‘Raudoniai’ | RA | Lithuania | V00015 |
| ‘Fredos taurieji’ | FT | Lithuania | V00012 |
| ‘Fredos derlingieji’ | FD | Lithuania | V00011 |
| ‘Kauno ankstyvieji’ | KA | Lithuania | V00014 |
| ‘Kauno gražieji’ | KG | Lithuania | V00013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanius, Ž.; Dūdėnas, M.; Kaškonienė, V.; Stankevičius, M.; Skrzydlewska, E.; Drevinskas, T.; Ragažinskienė, O.; Obelevičius, K.; Maruška, A. Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods. Molecules 2022, 27, 2705. https://doi.org/10.3390/molecules27092705
Stanius Ž, Dūdėnas M, Kaškonienė V, Stankevičius M, Skrzydlewska E, Drevinskas T, Ragažinskienė O, Obelevičius K, Maruška A. Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods. Molecules. 2022; 27(9):2705. https://doi.org/10.3390/molecules27092705
Chicago/Turabian StyleStanius, Žydrūnas, Mantas Dūdėnas, Vilma Kaškonienė, Mantas Stankevičius, Elżbieta Skrzydlewska, Tomas Drevinskas, Ona Ragažinskienė, Kęstutis Obelevičius, and Audrius Maruška. 2022. "Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods" Molecules 27, no. 9: 2705. https://doi.org/10.3390/molecules27092705
APA StyleStanius, Ž., Dūdėnas, M., Kaškonienė, V., Stankevičius, M., Skrzydlewska, E., Drevinskas, T., Ragažinskienė, O., Obelevičius, K., & Maruška, A. (2022). Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods. Molecules, 27(9), 2705. https://doi.org/10.3390/molecules27092705