Synthesis and Characterization of Diketopyrrolopyrrole-Based Aggregation-Induced Emission Nanoparticles for Bioimaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
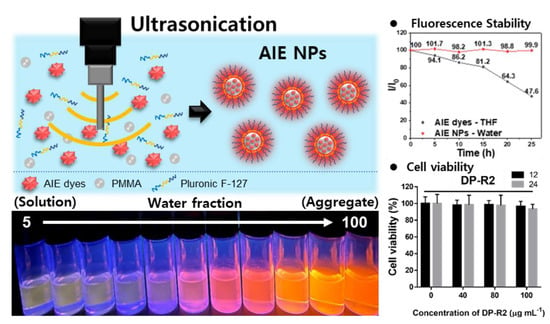
2.2. Preparation of AIE NPs
2.3. Characterization of AIE NP Size and Morphology
2.4. Optical Property Characterization
2.5. Cell Viability
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Geffroy, B.; Le Roy, P.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572–582. [Google Scholar] [CrossRef]
- Adil, M.A.; Zhang, J.; Wang, Y.; Yu, J.; Yang, C.; Lu, G.; Wei, Z. Regulating the phase separation of ternary organic solar cells via 3D architectured AIE molecules. Nano Energy 2020, 68, 104271. [Google Scholar] [CrossRef]
- Varnavski, O.; Pinsky, B.; Goodson III, T. Entangled photon excited fluorescence in organic materials: An ultrafast coincidence detector. J. Phys. Chem. Lett. 2017, 8, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Y.; Xu, B.; Tian, W. Fluorescent nanoparticles based on AIE fluorogens for bioimaging. Nanoscale 2016, 8, 2471–2487. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Tang, B.Z. AIE luminogens for bioimaging and theranostics: From organelles to animals. Chem 2017, 3, 56–91. [Google Scholar] [CrossRef] [Green Version]
- Kwok, R.T.; Leung, C.W.; Lam, J.W.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Kishimoto, K.; Urade, R.; Ogawa, T.; Moriyama, T. Nondestructive quantification of neutral lipids by thin-layer chromatography and laser-fluorescent scanning: Suitable methods for “lipidome” analysis. Biochem. Biophys. Res. Commun. 2001, 281, 657–662. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and exciplexes of conjugated polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef]
- Tang, B.Z.; Zhan, X.; Yu, G.; Lee, P.P.S.; Liu, Y.; Zhu, D. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, J.W.; Kwok, R.T.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Gu, K.; Zhu, W.-H.; Peng, X. Enhancement strategies of targetability, response and photostability for in vivo bioimaging. Sci. China Chem. 2019, 62, 189–198. [Google Scholar] [CrossRef]
- Lu, W.; Gong, X.; Yang, Z.; Zhang, Y.; Hu, Q.; Shuang, S.; Dong, C.; Choi, M.M. High-quality water-soluble luminescent carbon dots for multicolor patterning, sensors, and bioimaging. RSC Adv. 2015, 5, 16972–16979. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, K.; Zhao, L.; Li, C.; Bu, W.; Shen, Y.; Gu, Z.; Chang, B.; Zheng, C.; Lin, C. Aspirin-based carbon dots, a good biocompatibility of material applied for bioimaging and anti-inflammation. ACS Appl. Mater. Interfaces 2016, 8, 32706–32716. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zeng, X.; Xiao, Y.; Tang, L.; Nong, J.; Liu, Y.; Zhou, H.; Ding, B.; Xu, F.; Tong, H. Novel near-infrared II aggregation-induced emission dots for in vivo bioimaging. Chem. Sci. 2019, 10, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Sharma, A.; Malairaman, U.; Singh, R.R. Proficient surface modification of CdSe quantum dots for highly luminescent and biocompatible probes for bioimaging: A comparative experimental investigation. J. Lumin. 2018, 199, 174–182. [Google Scholar] [CrossRef]
- Hezinger, A.; Teßmar, J.; Göpferich, A. Polymer coating of quantum dots–a powerful tool toward diagnostics and sensorics. Eur. J. Pharm. Biopharm. 2008, 68, 138–152. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Hong, Y.; Tang, B.Z. Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging. Mater. Horiz. 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Polavarapu, L.; Manna, M.; Xu, Q.-H. Biocompatible glutathione capped gold clusters as one-and two-photon excitation fluorescence contrast agents for live cells imaging. Nanoscale 2011, 3, 429–434. [Google Scholar] [CrossRef]
- He, H.; Xie, C.; Ren, J. Nonbleaching fluorescence of gold nanoparticles and its applications in cancer cell imaging. Anal. Chem. 2008, 80, 5951–5957. [Google Scholar] [CrossRef]
- Huang, Y.; Fuksman, L.; Zheng, J. Luminescence mechanisms of ultrasmall gold nanoparticles. Dalton Trans. 2018, 47, 6267–6273. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Fan, J.; Sun, W.; Cao, J.; Hu, C.; Peng, X. A near-infrared fluorescent probe for selective detection of HClO based on Se-sensitized aggregation of heptamethine cyanine dye. Chem. Commun. 2014, 50, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Wang, Y.; Li, Y.; Zhu, W.; James, T.D. A near-infrared colorimetric fluorescent chemodosimeter for the detection of glutathione in living cells. Chem. Commun. 2014, 50, 1751–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Miyazaki, S.; Takeuchi, S.; Yokouchi, C.; Takada, M. Pluronic F-127 gels as a vehicle for topical administration of anticancer agents. Chem. Pharm. Bull. 1984, 32, 4205–4208. [Google Scholar] [CrossRef] [Green Version]
- Vollrath, A.; Pretzel, D.; Pietsch, C.; Perevyazko, I.; Schubert, S.; Pavlov, G.M.; Schubert, U.S. Preparation, cellular internalization, and biocompatibility of highly fluorescent PMMA nanoparticles. Macromol. Rapid Commun. 2012, 33, 1791–1797. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Enhanced photo-stability, thermal-stability and aqueous-stability of indocyanine green in polymeric nanoparticulate systems. J. Photochem. Photobiol. B Biol. 2004, 74, 29–38. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, M.; Gong, P.; Jia, D.; Zhang, P.; Shi, B.; Sheng, Z.; Ma, Y.; Cai, L. Indocyanine green-loaded biodegradable tumor targeting nanoprobes for in vitro and in vivo imaging. Biomaterials 2012, 33, 5603–5609. [Google Scholar] [CrossRef]
- Hwang, T.G.; Kim, G.Y.; Han, J.I.; Kim, S.; Kim, J.P. Enhancement of Lipid Productivity of Chlorella sp. Using Light-Converting Red Fluorescent Films Based on Aggregation-Induced Emission. ACS Sustain. Chem. Eng. 2020, 8, 15888–15897. [Google Scholar] [CrossRef]
- Guo, E.Q.; Ren, P.H.; Zhang, Y.L.; Zhang, H.C.; Yang, W.J. Diphenylamine end-capped 1,4-diketo-3,6-diphenylpyrrolo [3,4-c] pyrrole (DPP) derivatives with large two-photon absorption cross-sections and strong two-photon excitation red fluorescence. Chem. Commun. 2009, 39, 5859–5861. [Google Scholar] [CrossRef]
- Jiang, T.; Li, D.; Hang, Y.; Gao, Y.; Zhang, H.; Zhao, X.; Li, X.; Li, B.; Qian, J.; Hua, J. Tetraphenylethene end-capped diketopyrrolopyrrole fluorogens with AIE and large two-photon absorption cross-sections features and application in bioimaging. Dye. Pigment. 2016, 133, 201–213. [Google Scholar] [CrossRef]
- Hwang, T.G.; Kim, G.-Y.; Han, J.-I.; Park, J.M.; Kim, J.P. Highly efficient light-converting films based on diketopyrrolopyrrole with deep-red aggregation-induced emission for enhancing the lipid productivity of Chlorella sp. Sustain. Energy Fuels 2021, 5, 5205–5215. [Google Scholar] [CrossRef]
- Zhang, G.-G.; Xu, S.-L.; Xiong, Y.-H.; Duan, H.; Chen, W.-Y.; Li, X.-M.; Yuan, M.-F.; Lai, W.-H. Ultrabright fluorescent microsphere and its novel application for improving the sensitivity of immunochromatographic assay. Biosens. Bioelectron. 2019, 135, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Liu, Q.; Li, Z.; Yan, H.; Ji, C.; Duan, J.; Liu, Z. Aggregation-induced emission (AIE) of pyridyl-enamido-based organoboron luminophores. Chem. Commun. 2015, 51, 784–787. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, S.T.; Lam, J.W.; Kwok, R.T.; Qian, J.; Tang, B.Z. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2020, 11, 1255. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Yang, C.; Qin, J. An aggregation-induced blue shift of emission and the self-assembly of nanoparticles from a novel amphiphilic oligofluorene. Chem. Commun. 2008, 47, 6303–6305. [Google Scholar] [CrossRef]
- Yang, X.; Lu, R.; Zhou, H.; Xue, P.; Wang, F.; Chen, P.; Zhao, Y. Aggregation-induced blue shift of fluorescence emission due to suppression of TICT in a phenothiazine-based organogel. J. Colloid Interface Sci. 2009, 339, 527–532. [Google Scholar] [CrossRef]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and characterization of biogenic tellurium nanoparticles by using Penicillium chrysogenum PTCC 5031: A novel approach in gold biotechnology. Iran. J. Pharm. Res. 2018, 17 (Suppl. S2), 87. [Google Scholar]











| Sample | AIE Dye (DP-O) (mg/mL) | Pluronic F-127 (mg) | PMMA (mg) | SDS Solution (%, w/w) |
|---|---|---|---|---|
| Run 1 | 0.5 | 25.0 | 50.0 | 0.250 |
| Run 2 | 12.5 | 50.0 | 0.250 | |
| Run 3 | 25.0 | 0 | 0.250 | |
| Run 4 | 25.0 | 25.0 | 0.250 | |
| Run 5 | 25.0 | 50.0 | 0.125 | |
| Run 6 | 25.0 | 50.0 | 0.375 | |
| Run 7 | 25.0 | 50.0 | 0.500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Park, J.; Jang, S.H.; Lee, S.Y.; Seong, J.; Jung, J.W.; Kim, K.; Hwang, T.G.; Choi, J. Synthesis and Characterization of Diketopyrrolopyrrole-Based Aggregation-Induced Emission Nanoparticles for Bioimaging. Molecules 2022, 27, 2984. https://doi.org/10.3390/molecules27092984
Lee G, Park J, Jang SH, Lee SY, Seong J, Jung JW, Kim K, Hwang TG, Choi J. Synthesis and Characterization of Diketopyrrolopyrrole-Based Aggregation-Induced Emission Nanoparticles for Bioimaging. Molecules. 2022; 27(9):2984. https://doi.org/10.3390/molecules27092984
Chicago/Turabian StyleLee, Geonho, Jongwook Park, Seong Hyun Jang, Sang Yoon Lee, Jihyun Seong, Jae Woong Jung, Kyobum Kim, Tae Gyu Hwang, and Jun Choi. 2022. "Synthesis and Characterization of Diketopyrrolopyrrole-Based Aggregation-Induced Emission Nanoparticles for Bioimaging" Molecules 27, no. 9: 2984. https://doi.org/10.3390/molecules27092984
APA StyleLee, G., Park, J., Jang, S. H., Lee, S. Y., Seong, J., Jung, J. W., Kim, K., Hwang, T. G., & Choi, J. (2022). Synthesis and Characterization of Diketopyrrolopyrrole-Based Aggregation-Induced Emission Nanoparticles for Bioimaging. Molecules, 27(9), 2984. https://doi.org/10.3390/molecules27092984