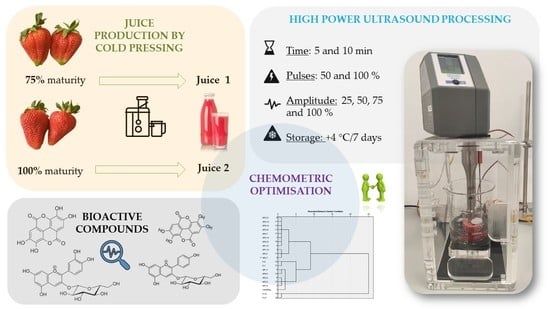
A Chemometric Investigation on the Functional Potential in High Power Ultrasound (HPU) Processed Strawberry Juice Made from Fruits Harvested at two Stages of Ripeness
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Use of Chemometrics for the Evaluation of HPU Processing
2.2. The Changes of Bioactive Compounds in Strawberry Juices under HPU Processing and Storage
2.3. Optimization of HPU Parameters for Strawberry Juice Treatment
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Production of Strawberry Juice
3.3. High Power Ultrasound (HPU) Processing of Strawberry Juice
3.4. Extraction of Bioactive Compounds
3.5. Determination of Total Phenolic Content (TPC)
3.6. Determination of Total Monomeric Anthocyanins (ANT)
3.7. Determination of Total Hydroxycinnamic Acids (HCA)
3.8. Determination of Total Flavonols (TF)
3.9. Determination of Condensed Tannins (CT)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2013, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2017, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Tomašević, I.; Putnik, P.; Valjak, F.; Pavlić, B.; Šojić, B.; Bebek Markovinović, A.; Bursać Kovačević, D. 3d printing as novel tool for fruit-based functional food production. Curr. Opin. Food Sci. 2021, 41, 138–145. [Google Scholar] [CrossRef]
- Žuntar, I.; Petric, Z.; Bursać Kovačević, D.; Putnik, P. Safety of probiotics: Functional fruit beverages and nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence-based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [Green Version]
- Kowaleski, J.; Quast, L.B.; Steffens, J.; Lovato, F.; Rodrigues dos Santos, L.; Zambiazi da Silva, S.; Maschio de Souza, D.; Felicetti, M.A. Functional yogurt with strawberries and chia seeds. Food Biosci. 2020, 37, 100726. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimarães, J.T.; Capozzi, V.; Russo, P.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Raices, R.S.L.; Silva, M.C.; et al. Novel milk–juice beverage with fermented sheep milk and strawberry (fragaria × ananassa): Nutritional and functional characterization. J. Dairy Sci. 2019, 102, 10724–10736. [Google Scholar] [CrossRef] [Green Version]
- Korus, J.; Juszczak, L.; Ziobro, R.; Witczak, M.; Grzelak, K.; SÓJka, M. Defatted strawberry and blackcurrant seeds as functional ingredients of gluten-free bread. J. Texture Stud. 2012, 43, 29–39. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, X.; Chen, H.; Liu, Y.; Xiao, Y.; Chen, H.; Tang, Z.; Li, Q.; Yao, H. Evaluation of a strawberry fermented beverage with potential health benefits. PeerJ 2021, 9, e11974. [Google Scholar] [CrossRef]
- Guo, L.; Li, K.; Kang, J.S.; Kang, N.J.; Son, B.G.; Choi, Y.W. Strawberry fermentation with Cordyceps militaris has anti-adipogenesis activity. Food Biosci. 2020, 35, 100576. [Google Scholar] [CrossRef]
- Özbek, T.; Şahin-Yeşilçubuk, N.; Demirel, B. Quality and nutritional value of functional strawberry marmalade enriched with chia seed (Salvia hispanica L.). J. Food Qual. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.R.; Mohamed, R.M.; Abedelmaksoud, T.G. Functional strawberry and red beetroot jelly candies rich in fibers and phenolic compounds. Food Syst. 2021, 4, 82–88. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Moreira, M.R.; Agüero, M.V. Improving quality parameters of functional strawberry juices: Optimization of prebiotic fiber enrichment and geraniol treatment. Food Bioprocess Technol. 2018, 11, 2110–2124. [Google Scholar] [CrossRef]
- Soliman, Z.; Mekky, T.M.; Zeid, M.H. Production of functional drinks from blood orange and strawberry. J. Home Econ. 2015, 25, 131–148. [Google Scholar]
- Moscovici Joubran, A.; Katz, I.H.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. The effect of pressure level and cycling in high-pressure homogenization on physicochemical, structural and functional properties of filtered and non-filtered strawberry nectar. Innov. Food Sci. Emerg. Technol. 2019, 57, 102203. [Google Scholar] [CrossRef]
- Santhi, V.P.; Sriramavaratharajan, V.; Murugan, R.; Masilamani, P.; Gurav, S.S.; Sarasu, V.P.; Parthiban, S.; Ayyanar, M. Edible fruit extracts and fruit juices as potential source of antiviral agents: A review. J. Food Meas. Charact. 2021, 15, 5181–5190. [Google Scholar] [CrossRef]
- Available online: https://www.grandviewresearch.com/industry-analysis/fruit-vegetable-juice-market (accessed on 14 September 2022).
- Šamec, D.; Maretić, M.; Lugarić, I.; Mešić, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016, 194, 828–834. [Google Scholar] [CrossRef]
- Voća, S.; Duralija, B.; Družić, J.; Skendrović-Babojelić, M.; Dobričević, N.; Čmelik, Z. Influence of cultivation systems on physical and chemical composition of strawberry fruits cv. Elsanta. Agric. Conspec. Sci. 2006, 71, 171–174. [Google Scholar]
- Azam, M.; Ejaz, S.; Naveed Ur Rehman, R.; Khan, M.; Qadri, R. Postharvest quality management of strawberries. In Strawberry—Pre- and Post-Harvest Management Techniques for Higher Fruit Quality; Toshiki, A., Md, A., Eds.; IntechOpen: London, UK, 2019; pp. 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bebek Markovinović, A.; Putnik, P.; Duralija, B.; Krivohlavek, A.; Ivešić, M.; Mandić Andačić, I.; Palac Bešlić, I.; Pavlić, B.; Lorenzo, J.M.; Bursać Kovačević, D. Chemometric valorization of strawberry (fragaria × ananassa duch.) cv. ‘Albion’ for the production of functional juice: The impact of physicochemical, toxicological, sensory, and bioactive value. Foods 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Bursać Kovačević, D.; Brdar, D.; Fabečić, P.; Barba, F.J.; Lorenzo, J.M.; Putnik, P. Strategies to achieve a healthy and balanced diet: Fruits and vegetables as a natural source of bioactive compounds. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Barba, F.J., Putnik, P., Bursać Kovačević, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–88. [Google Scholar]
- Teribia, N.; Buvé, C.; Bonerz, D.; Aschoff, J.; Hendrickx, M.; Loey, A.V. Effect of cultivar, pasteurization and storage on the volatile and taste compounds of strawberry puree. LWT 2021, 150, 112007. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Vahčić, N.; Babojelić, M.S.; Levaj, B. Influences of organically and conventionally grown strawberry cultivars on anthocyanins content and color in purees and low-sugar jams. Food Chem. 2015, 181, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Patz, C.-D.; Andlauer, W.; Dietrich, H.; Ludwig, M. Influence of processing on quality parameters of strawberries. J. Agric. Food Chem. 2008, 56, 9484–9489. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Aguayo, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in quality attributes throughout storage of strawberry juice processed by high-intensity pulsed electric fields or heat treatments. LWT—Food Sci. Technol. 2009, 42, 813–818. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Chemat, F.; Zille, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Bursać Kovačević, D.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and conventional valorizations of grape seeds from winery by-products as sustainable source of lipophilic antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019, 58, 104609. [Google Scholar] [CrossRef]
- Bhat, R.; Goh, K.M. Sonication treatment convalesce the overall quality of hand-pressed strawberry juice. Food Chem. 2017, 215, 470–476. [Google Scholar] [CrossRef]
- Tomadoni, B.; Cassani, L.; Viacava, G.; Moreira, M.D.R.; Ponce, A. Effect of ultrasound and storage time on quality attributes of strawberry juice. J. Food Process Eng. 2017, 40, e12533. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Yildiz, G.; Aadil, R.M. Comparison of high temperature-short time and sonication on selected parameters of strawberry juice during room temperature storage. J. Food Sci. Technol. 2019, 57, 1462–1468. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Putnik, P.; Stulić, V.; Batur, L.; Duralija, B.; Pavlić, B.; Vukušić Pavičić, T.; Herceg, Z.; Bursać Kovačević, D. The application and optimization of hipef technology in the processing of juice from strawberries harvested at two stages of ripeness. Foods 2022, 11, 1997. [Google Scholar] [CrossRef]
- Aubert, C.; Bruaut, M.; Chalot, G.; Cottet, V. Impact of maturity stage at harvest on the main physicochemical characteristics, the levels of vitamin c, polyphenols and volatiles and the sensory quality of gariguette strawberry. Eur. Food Res. Technol. 2020, 247, 37–49. [Google Scholar] [CrossRef]
- Cruz-Cansino, N.D.S.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanis-Garcia, E.; Ariza-Ortega, J.A.; Manriquez-Torres, J.D.J.; Jaramillo-Bustor, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochem. 2015, 27, 277–286. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef]
- Kataoka, H. New trends in sample preparation for analysis of plant-derived medicines. Curr. Org. Chem. 2010, 14, 1698–1713. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of high-power ultrasounds during red wine vinification. Int. J. Food Sci. Technol. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- Plaza, E.G.; Jurado, R.; Iniesta, J.A.; Bautista-Ortín, A.B. High power ultrasounds: A powerful, non-thermal and green technique for improving the phenolic extraction from grapes to must during red wine vinification. BIO Web Conf. 2019, 12, 02001. [Google Scholar] [CrossRef]
- Wei, M.; Wang, H.; Ma, T.; Ge, Q.; Fang, Y.; Sun, X. Comprehensive utilization of thinned unripe fruits from horticultural crops. Foods 2021, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Pradas, I.; Medina, J.J.; Ortiz, V.; Moreno-Rojas, J.M. ‘Fuentepina’ and ‘amiga’, two new strawberry cultivars: Evaluation of genotype, ripening and seasonal effects on quality characteristics and health-promoting compounds. J. Berry Res. 2015, 5, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Muche, B.M.; Speers, R.A.; Rupasinghe, H.P.V. Storage temperature impacts on anthocyanins degradation, color changes and haze development in juice of “merlot” and “ruby” grapes (Vitis vinifera). Front. Nutr. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wang, Z.; Dong, L.; Huang, F.; Zhang, R.; Jia, X.; Wu, G.; Zhang, M. Impact of thermal processing and storage temperature on the phenolic profile and antioxidant activity of different varieties of lychee juice. Lwt 2019, 116, 108578. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Bilobrk, J.; Buntić, B.; Bosiljkov, T.; Karlović, S.; Rocchetti, G.; Lucini, L.; Barba, F.J.; Lorenzo, J.M.; Putnik, P. High-power ultrasound altered the polyphenolic content and antioxidant capacity in cloudy apple juice during storage. J. Food Process. Preserv. 2019, 43, e14023. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Zorba, N.N.D.; ÖZcan, G. Microbial inactivation and physicochemical properties of ultrasound processed pomegranate juice. J. Food Prot. 2015, 78, 531–539. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Cullen, P.J. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J. Agric. Food Chem. 2008, 56, 10071–10077. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Brunton, N.; Cullen, P.J. Stability of anthocyanins and ascorbic acid in sonicated strawberry juice during storage. Eur. Food Res. Technol. 2008, 228, 717–724. [Google Scholar] [CrossRef]
- Suslick, K.S. The chemical effects of ultrasound. Sci. Am. 1989, 260, 80–86. [Google Scholar] [CrossRef]
- Lukić, K.; Brnčić, M.; Ćurko, N.; Tomašević, M.; Valinger, D.; Denoya, G.I.; Barba, F.J.; Ganić, K.K. Effects of high power ultrasound treatments on the phenolic, chromatic and aroma composition of young and aged red wine. Ultrason. Sonochem. 2019, 59, 104725. [Google Scholar] [CrossRef]
- Celotti, E.; Stante, S.; Ferraretto, P.; Román, T.; Nicolini, G.; Natolino, A. High power ultrasound treatments of red young wines: Effect on anthocyanins and phenolic stability indices. Foods 2020, 9, 1344. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; de Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. Power ultrasound processing of cantaloupe melon juice: Effects on quality parameters. Food Res. Int. 2012, 48, 41–48. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Almeida, F.D.L.; de Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. High-intensity ultrasound processing of pineapple juice. Food Bioprocess Technol. 2011, 6, 997–1006. [Google Scholar] [CrossRef]
- Jiang, B.; Mantri, N.; Hu, Y.; Lu, J.; Jiang, W.; Lu, H. Evaluation of bioactive compounds of black mulberry juice after thermal, microwave, ultrasonic processing, and storage at different temperatures. Food Sci. Technol. Int. 2014, 21, 392–399. [Google Scholar] [CrossRef]
- Cassani, L.; Gerbino, E.; Moreira, M.d.R.; Gómez-Zavaglia, A. Influence of non-thermal processing and storage conditions on the release of health-related compounds after in vitro gastrointestinal digestion of fiber-enriched strawberry juices. J. Funct. Foods 2018, 40, 128–136. [Google Scholar] [CrossRef]
- Stübler, A.-S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of pilot-scale processing (thermal, PEF, HPP) on the stability and bioaccessibility of polyphenols and proteins in mixed protein- and polyphenol-rich juice systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Nadeem, M.; Ubaid, N.; Qureshi, T.M.; Munir, M.; Mehmood, A. Effect of ultrasound and chemical treatment on total phenol, flavonoids and antioxidant properties on carrot-grape juice blend during storage. Ultrason. Sonochem. 2018, 45, 1–6. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Margean, A.; Lupu, M.I.; Alexa, E.; Padureanu, V.; Canja, C.M.; Cocan, I.; Negrea, M.; Calefariu, G.; Poiana, M.-A. An overview of effects induced by pasteurization and high-power ultrasound treatment on the quality of red grape juice. Molecules 2020, 25, 1669. [Google Scholar] [CrossRef] [Green Version]
- Jabbar, S.; Abid, M.; Hu, B.; Hashim, M.M.; Lei, S.; Wu, T.; Zeng, X. Exploring the potential of thermosonication in carrot juice processing. J. Food Sci. Technol. 2015, 52, 7002–7013. [Google Scholar] [CrossRef]
- Dündar, B.; Ağçam, E.; Akyıldız, A. Optimization of thermosonication conditions for cloudy strawberry nectar with using of critical quality parameters. Food Chem. 2019, 276, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wahia, H.; Zhou, C.; Sarpong, F.; Mustapha, A.T.; Liu, S.; Yu, X.; Li, C. Simultaneous optimization of Alicyclobacillus acidoterrestris reduction, pectin methylesterase inactivation, and bioactive compounds enhancement affected by thermosonication in orange juice. J. Food Process. Preserv. 2019, 43, e14180. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Bermúdez-Aguirre, D.; Martínez-Flores, H.E.; Garnica-Romo, M.G.; Sablani, S.; Tang, J.; Barbosa-Cánovas, G.V. Combined effect of ultrasound and mild temperatures on the inactivation of E. coli in fresh carrot juice and changes on its physicochemical characteristics. J. Food Sci. 2017, 82, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.-G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (hpp) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]

| Maturity | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|
| 75% | 93.62 ± 1.99 | 8.99 ± 0.13 | 10.56 ± 0.68 | 1.77 ± 0.68 | 70.65 ± 1.53 |
| 100% | 96.18 ± 3.81 | 17.84 ± 0.31 | 16.66 ± 0.71 | 2.44 ± 0.82 | 88.75 ± 1.60 |
| Bioactive Compound | Treatment | Mean Rank | Chi-Square | Significance |
|---|---|---|---|---|
| TPC | Control HPU | 2.50 32.5 | 2.528 | 0.11 |
| ANT | Control HPU | 2.50 32.5 | 1.838 | 0.18 |
| HCA | Control HPU | 2.50 32.5 | 0.633 | 0.43 |
| FL | Control HPU | 2.50 32.5 | 1.416 | 0.23 |
| CT | Control HPU | 2.50 32.5 | 4.134 | 0.04 |
| Variable | n | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|---|
| Maturity | 4 | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.06 ‡ | p ≤ 0.01 † |
| 75% | 4 | 103.1 ± 1.26 a | 8.84 ± 0.09 b | 11.71 ± 0.24 b | 2.25 ± 0.55 a | 75.56 ± 0.91 b |
| 100% | 4 | 90.73 ± 1.26 b | 17.21 ± 0.09 a | 17.76 ± 0.24 a | 4.27 ± 0.55 a | 86.54 ± 0.91 a |
| Storage | p = 0.09 ‡ | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.02 † | p = 0.10 ‡ | |
| 0 days | 4 | 94.90 ± 1.26 a | 13.42 ± 0.09 a | 13.61 ± 0.24 b | 2.11 ± 0.55 b | 79.70 ± 0.91 a |
| 7 days | 4 | 98.94 ± 1.26 a | 12.63 ± 0.09 b | 15.86 ± 0.24 a | 4.41 ± 0.55 a | 82.39 ± 0.91 a |
| Dataset average | 8 | 96.92 ± 0.89 | 13.023 ± 0.09 | 14.74 ± 0.17 | 3.26 ± 0.39 | 81.05 ± 0.64 |
| Variable | n | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|---|
| Maturity | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 75% | 64 | 92.61 ± 0.49 a | 8.22 ± 0.03 b | 10.66 ± 0.08 b | 2.06 ± 0.11 b | 67.23 ± 0.21 b |
| 100% | 64 | 78.59 ± 0.49 b | 15.02 ± 0.03 a | 14.92 ± 0.08 a | 2.94 ± 0.11 a | 70.48 ± 0.21 a |
| Amplitude | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.91 ‡ | p ≤ 0.01 † | |
| 25% | 32 | 89.08 ± 0.69 a | 12.14 ± 0.04 a | 13.24 ± 0.12 a | 2.57 ± 0.16 a | 73.56 ± 0.30 a |
| 50% | 32 | 86.22 ± 0.69 b | 11.48 ± 0.04 b | 12.83 ± 0.12 b | 2.54 ± 0.16 a | 68.87 ± 0.30 b |
| 75% | 32 | 82.16 ± 0.69 c | 11.60 ± 0.04 c | 12.66 ± 0.12 b,c | 2.42 ± 0.16 a | 67.76 ± 0.30 c |
| 100% | 32 | 84.94 ± 0.69 b | 11.27 ± 0.04 d | 12.44 ± 0.12 c | 2.47 ± 0.16 a | 65.23 ± 0.30 d |
| Pulse | p = 0.02 † | p ≤ 0.01 † | p = 0.19 ‡ | p = 0.05 † | p ≤ 0.01 † | |
| 50% | 64 | 84.88 ± 0.49 b | 11.71 ± 0.03 a | 12.86 ± 0.08 a | 2.66 ± 0.12 a | 69.30 ± 0.21 a |
| 100% | 64 | 86.32 ± 0.49 a | 11.53 ± 0.03 b | 12.72 ± 0.08 a | 2.34 ± 0.12 b | 68.41 ± 0.21 b |
| Treatment time | p = 0.09 ‡ | p ≤ 0.01 † | p = 0.49 ‡ | p ≤ 0.01 † | p ≤ 0.01 † | |
| 5 min | 64 | 84.97 ± 0.49 a | 11.75 ± 0.03 a | 12.75 ± 0.08 a | 2.75 ± 0.12 a | 69.38 ± 0.21 a |
| 10 min | 64 | 86.23 ± 0.49 a | 11.50 ± 0.03 b | 12.83 ± 0.08 a | 2.26 ± 0.12 b | 68.33 ± 0.21 b |
| Storage | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.54 ‡ | |
| 0 days | 64 | 87.25 ± 0.49 a | 12.03 ± 0.03 a | 12.41 ± 0.08 b | 1.81 ± 0.11 b | 68.76 ± 0.21 a |
| 7 days | 64 | 83.95 ± 0.49 b | 11.21 ± 0.03 b | 13.18 ± 0.08 a | 3.20 ± 0.11 a | 68.95 ± 0.21 a |
| Dataset average | 128 | 85.60 ± 0.34 | 11.62 ± 0.02 | 12.79 ± 0.05 | 2.50 ± 0.08 | 68.55 ± 0.15 |
| Amplitudes (%) | Pulse (%) | Treatment Time (min) | Energy (Wh) | Power (W) | Frequency (kHz) | ΔT (°C) |
|---|---|---|---|---|---|---|
| 25.0 | 50.0 | 10.0 | 2.9 | 40.0 | 23.7 | 4.0 |
| 25.0 | 50.0 | 5.0 | 1.5 | 42.0 | 23.7 | 8.0 |
| 25.0 | 50.0 | 10.0 | 3.0 | 42.0 | 23.6 | 12.0 |
| 50.0 | 50.0 | 10.0 | 3.5 | 78.0 | 23.7 | 14.0 |
| 50.0 | 50.0 | 5.0 | 1.9 | 71.0 | 23.7 | 15.0 |
| 50.0 | 50.0 | 10.0 | 3.7 | 71.0 | 23.8 | 16.0 |
| 25.0 | 50.0 | 5.0 | 1.5 | 45.0 | 23.6 | 17.0 |
| 25.0 | 100.0 | 5.0 | 3.5 | 44.0 | 23.8 | 18.0 |
| 66.7 | 66.7 | 6.7 | 3.7 | 78.7 | 23.7 | 19.0 |
| 50.0 | 100.0 | 5.0 | 6.6 | 90.0 | 23.8 | 21.0 |
| 37.5 | 75.0 | 7.5 | 4.4 | 52.5 | 23.7 | 22.0 |
| 25.0 | 100.0 | 5.0 | 3.8 | 38.0 | 23.6 | 23.0 |
| 75.0 | 50.0 | 5.0 | 2.2 | 85.0 | 23.8 | 25.0 |
| 100.0 | 50.0 | 7.5 | 3.1 | 88.0 | 23.8 | 26.0 |
| 75.0 | 50.0 | 10.0 | 4.2 | 85.0 | 23.8 | 29.0 |
| 75.0 | 100.0 | 5.0 | 10.0 | 133.0 | 23.8 | 31.0 |
| 75.0 | 50.0 | 10.0 | 4.4 | 74.0 | 23.8 | 33.0 |
| 75.0 | 75.0 | 10.0 | 7.9 | 76.0 | 23.8 | 34.0 |
| 100.0 | 100.0 | 5.0 | 11.5 | 177.0 | 23.8 | 35.0 |
| 50.0 | 100.0 | 5.0 | 6.6 | 93.0 | 23.8 | 36.0 |
| 100.0 | 100.0 | 5.0 | 11.1 | 166.0 | 23.8 | 37.0 |
| 50.0 | 100.0 | 10.0 | 12.8 | 90.0 | 23.8 | 46.0 |
| 75.0 | 100.0 | 7.5 | 13.5 | 129.0 | 23.8 | 49.0 |
| 100.0 | 100.0 | 10.0 | 20.8 | 177.0 | 23.8 | 50.0 |
| 75.0 | 100.0 | 10.0 | 16.4 | 128.0 | 23.8 | 51.0 |
| 100.0 | 100.0 | 10.0 | 19.9 | 176.0 | 23.8 | 54.0 |
| ΔT (°C) | Amplitudes (%) | Pulse (%) | Treatment Time (min) | Energy (Wh) | Power (W) | Frequency (kHz) | |
|---|---|---|---|---|---|---|---|
| ΔT (°C) | 1 | 0.59 † | 0.61 † | 0.22 ‡ | 0.85 † | 0.76 † | 0.52 † |
| Amplitudes (%) | 1 | 0 | 0 | 0.39 † | 0.79 † | 0.65 † | |
| Pulse (%) | 1 | 0 | 0.73 † | 0.44 † | 0.13 ‡ | ||
| Treatment time (min) | 1 | 0.37 † | −0.02 ‡ | −0.10 ‡ | |||
| Energy (Wh) | 1 | 0.76 † | 0.31 ‡ | ||||
| Power (W) | 1 | 0.58 † | |||||
| Frequency (kHz) | 1 |
| Analytical Variable | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|
| Content (mg 100 mL−1) | 102.96 | 15.58 | 14.14 | 2.68 | 75.28 |
| Maturity (%) | 75 | 100 | 100 | 75 | 100 |
| ΔT (°C) | 4.0 | 35.41 | 36.77 | 4.0 | 4.0 |
| Sample | Juice | Storage (Days) | Treatment | Amplitude (%) | Pulse (%) | Treatment Time (min) |
|---|---|---|---|---|---|---|
| 1 | J1 | 0 | Control | / | / | / |
| 2 | J1 | 0 | HPU | 25 | 50 | 5 |
| 3 | J1 | 0 | HPU | 10 | ||
| 4 | J1 | 0 | HPU | 100 | 5 | |
| 5 | J1 | 0 | HPU | 10 | ||
| 6 | J1 | 0 | HPU | 50 | 50 | 5 |
| 7 | J1 | 0 | HPU | 10 | ||
| 8 | J1 | 0 | HPU | 100 | 5 | |
| 9 | J1 | 0 | HPU | 10 | ||
| 10 | J1 | 0 | HPU | 75 | 50 | 5 |
| 11 | J1 | 0 | HPU | 10 | ||
| 12 | J1 | 0 | HPU | 100 | 5 | |
| 13 | J1 | 0 | HPU | 10 | ||
| 14 | J1 | 0 | HPU | 100 | 50 | 5 |
| 15 | J1 | 0 | HPU | 10 | ||
| 16 | J1 | 0 | HPU | 100 | 5 | |
| 17 | J1 | 0 | HPU | 10 | ||
| 18 | J2 | 0 | Control | / | / | / |
| 19 | J2 | 0 | HPU | 25 | 50 | 5 |
| 20 | J2 | 0 | HPU | 10 | ||
| 21 | J2 | 0 | HPU | 100 | 5 | |
| 22 | J2 | 0 | HPU | 10 | ||
| 23 | J2 | 0 | HPU | 50 | 50 | 5 |
| 24 | J2 | 0 | HPU | 10 | ||
| 25 | J2 | 0 | HPU | 100 | 5 | |
| 26 | J2 | 0 | HPU | 10 | ||
| 27 | J2 | 0 | HPU | 75 | 50 | 5 |
| 28 | J2 | 0 | HPU | 10 | ||
| 29 | J2 | 0 | HPU | 100 | 5 | |
| 30 | J2 | 0 | HPU | 10 | ||
| 31 | J2 | 0 | HPU | 100 | 50 | 5 |
| 32 | J2 | 0 | HPU | 10 | ||
| 33 | J2 | 0 | HPU | 100 | 5 | |
| 34 | J2 | 0 | HPU | 10 | ||
| 35 | J1 | 7 | Control | / | / | / |
| 36 | J1 | 7 | HPU | 25 | 50 | 5 |
| 37 | J1 | 7 | HPU | 10 | ||
| 38 | J1 | 7 | HPU | 100 | 5 | |
| 39 | J1 | 7 | HPU | 10 | ||
| 40 | J1 | 7 | HPU | 50 | 50 | 5 |
| 41 | J1 | 7 | HPU | 10 | ||
| 42 | J1 | 7 | HPU | 100 | 5 | |
| 43 | J1 | 7 | HPU | 10 | ||
| 44 | J1 | 7 | HPU | 75 | 50 | 5 |
| 45 | J1 | 7 | HPU | 10 | ||
| 46 | J1 | 7 | HPU | 100 | 5 | |
| 47 | J1 | 7 | HPU | 10 | ||
| 48 | J1 | 7 | HPU | 100 | 50 | 5 |
| 49 | J1 | 7 | HPU | 10 | ||
| 50 | J1 | 7 | HPU | 100 | 5 | |
| 51 | J1 | 7 | HPU | 10 | ||
| 52 | J2 | 7 | Control | / | / | / |
| 53 | J2 | 7 | HPU | 25 | 50 | 5 |
| 54 | J2 | 7 | HPU | 10 | ||
| 55 | J2 | 7 | HPU | 100 | 5 | |
| 56 | J2 | 7 | HPU | 10 | ||
| 57 | J2 | 7 | HPU | 50 | 50 | 5 |
| 58 | J2 | 7 | HPU | 10 | ||
| 59 | J2 | 7 | HPU | 100 | 5 | |
| 60 | J2 | 7 | HPU | 10 | ||
| 61 | J2 | 7 | HPU | 75 | 50 | 5 |
| 62 | J2 | 7 | HPU | 10 | ||
| 63 | J2 | 7 | HPU | 100 | 5 | |
| 64 | J2 | 7 | HPU | 10 | ||
| 65 | J2 | 7 | HPU | 100 | 50 | 5 |
| 66 | J2 | 7 | HPU | 10 | ||
| 67 | J2 | 7 | HPU | 100 | 5 | |
| 68 | J2 | 7 | HPU | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebek Markovinović, A.; Putnik, P.; Bičanić, P.; Brdar, D.; Duralija, B.; Pavlić, B.; Milošević, S.; Rocchetti, G.; Lucini, L.; Bursać Kovačević, D. A Chemometric Investigation on the Functional Potential in High Power Ultrasound (HPU) Processed Strawberry Juice Made from Fruits Harvested at two Stages of Ripeness. Molecules 2023, 28, 138. https://doi.org/10.3390/molecules28010138
Bebek Markovinović A, Putnik P, Bičanić P, Brdar D, Duralija B, Pavlić B, Milošević S, Rocchetti G, Lucini L, Bursać Kovačević D. A Chemometric Investigation on the Functional Potential in High Power Ultrasound (HPU) Processed Strawberry Juice Made from Fruits Harvested at two Stages of Ripeness. Molecules. 2023; 28(1):138. https://doi.org/10.3390/molecules28010138
Chicago/Turabian StyleBebek Markovinović, Anica, Predrag Putnik, Paula Bičanić, Dora Brdar, Boris Duralija, Branimir Pavlić, Sanja Milošević, Gabriele Rocchetti, Luigi Lucini, and Danijela Bursać Kovačević. 2023. "A Chemometric Investigation on the Functional Potential in High Power Ultrasound (HPU) Processed Strawberry Juice Made from Fruits Harvested at two Stages of Ripeness" Molecules 28, no. 1: 138. https://doi.org/10.3390/molecules28010138
APA StyleBebek Markovinović, A., Putnik, P., Bičanić, P., Brdar, D., Duralija, B., Pavlić, B., Milošević, S., Rocchetti, G., Lucini, L., & Bursać Kovačević, D. (2023). A Chemometric Investigation on the Functional Potential in High Power Ultrasound (HPU) Processed Strawberry Juice Made from Fruits Harvested at two Stages of Ripeness. Molecules, 28(1), 138. https://doi.org/10.3390/molecules28010138