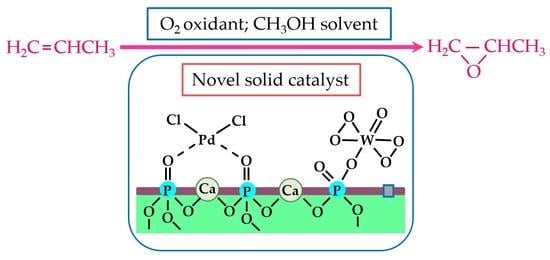
Immobilization of Peroxo-Heteropoly Compound and Palladium on Hydroxyapatite for the Epoxidation of Propylene by Molecular Oxygen in Methanol
Abstract
:1. Introduction
2. Results
2.1. FT-IR Spectra
2.2. Elemental Analyses
2.3. Epoxidation of Propylene by O2 in Methanol over Various Catalysts
2.4. Comparison of Catalytic Performance between Pd-PW-HAP and Pd+PW2
2.5. Stability of Solid Catalyst Pd-PW-HAP
2.6. Reusability of Solid Catalyst Pd-PW-HAP
2.7. XRD Patterns of Various Samples
2.8. TEM Images of Pd-PW-HAP before and after Reaction
2.9. EXAFS Functions of Pd-PW-HAP before and after Reaction
2.10. XPS Spectra of Pd-PW-HAP before and after Reaction
2.11. Solvent Effect in the Epoxidation of Propylene by O2 over Pd-PW-HAP
2.12. Co-Products from Methanol in the Epoxidation of Propylene by O2
3. Discussion
3.1. Synthesis Route of Pd-PW-HAP
3.2. Strong Points of Pd-PW-HAP
3.3. Active Pd Species in Pd-PW-HAP
3.4. Roles of Methanol in the Reaction
3.5. Reaction Mechanism for the Reaction
4. Materials and Methods
4.1. Reagents
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Reaction Procedure and Analyses
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Clerici, M.G.; Bellussi, C.; Romano, U. Synthesis of propylene oxide from propylene and hydrogen peroxide catalyzed by titanium silicalite. J. Catal. 1991, 129, 159–167. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, M.; Inaba, M.; Mimura, N. Selective oxidation of propylene to propylene oxide by molecular oxygen over Ti-Al-HMS catalysts. Catal. Lett. 2003, 89, 49–53. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, M.; Inaba, M.; Mimura, N. Syntheses of Ti- and Al-containing hexagonal mesoporous silicas for gas-phase epoxidation of propylene by molecular oxygen. Appl. Catal. A Gen. 2006, 309, 91–105. [Google Scholar] [CrossRef]
- Qi, C.; Okumura, M.; Akita, T.; Haruta, M. Vapor-phase epoxidation of propylene using H2/O2 mixture over gold catalysts supported on non-porous and mesoporous titania-silica: Effect of preparation conditions and pretreatments before reaction. Appl. Catal. A Gen. 2004, 263, 19–26. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Guo, Q.; Luo, Y.; Yang, L.; Wang, Y. Iron-catalysed propylene epoxidation by nitrous oxide: Dramatic shift of allylic oxidation to epoxidation by the modification with alkali metal salts. Chem. Commun. 2004, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Murata, K.; Inaba, M. Direct epoxidation of propylene by molecular oxygen over catalyst system containing palladium and peroxo-heteropoly compound in methanol. Chem. Commun. 2004, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Murata, K.; Inaba, M.; Mimura, N. Direct epoxidation of propylene by molecular oxygen over Pd(OAc)2-[(C6H13)4N]3{PO4[W(O)(O2)2]4}-CH3OH catalytic system. Appl. Catal. B Environ. 2005, 58, 51–59. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, Q.; Zhong, S. HPW/PAM catalyst for oxidative desulfurization-synthesis, characterization and mechanism study. Processes 2022, 10, 402. [Google Scholar] [CrossRef]
- Sedghi, R.; Heidari, B.; Shahmohamadi, H.; Zarshenas, P.; Varma, R.S. Pd nanocatalyst adorned on magnetic Chitosan@N-Heterocyclic carbene: Eco-compatible Suzuki cross-coupling reaction. Molecules 2019, 24, 3048. [Google Scholar] [CrossRef] [Green Version]
- Kuniyil, M.; Kumar, J.V.S.; Adil, S.F.; Shaik, M.R.; Khan, M.; Assal, M.E.; Siddiqui, M.R.H.; Al-Warthan, A. One-pot synthesized Pd@N-doped graphene: An efficient catalyst for Suzuki–Miyaura couplings. Catalysts 2019, 9, 469. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Epoxidation of propylene with molecular oxygen in methanol over a peroxo-heteropoly compound immobilized on palladium exchanged HMS. Green Chem. 2004, 6, 510–515. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Hanaoka, T.; Inaba, M.; Sakanishi, K. Syntheses of new peroxopolyoxometalates intercalated layered double hydroxides for propene epoxidation by molecular oxygen in methanol. J. Catal. 2007, 248, 277–287. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Misono, M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 13, 1141–1152. [Google Scholar] [CrossRef]
- Mizuno, N.; Yamaguchi, K.; Kamata, K. Epoxidation of olefins with hydrogen peroxide catalyzed by polyoxometalates. Coord. Chem. Rev. 2005, 249, 1944–1956. [Google Scholar] [CrossRef]
- Liu, Y.; Misono, M. Hydroisomerization of n-butane over platinum-promoted cesium hydrogen salt of 12-tungstophosphoric acid. Materials 2009, 2, 2319–2336. [Google Scholar] [CrossRef]
- Liu, Y.; Koyano, G.; Misono, M. Hydroisomerization of n-hexane and n-heptane over platinum promoted Cs2.5H0.5PW12O40 (Cs2.5) studied in comparison with several other solid acids. Top. Catal. 2000, 11, 239–246. [Google Scholar] [CrossRef]
- Liu, Y.; Na, K.; Misono, M. Skeletal isomerization of n-pentane over Pt-promoted cesium hydrogen salts of 12-tungstophosphoric acid. J. Mol. Catal. A Chem. 1999, 141, 145–153. [Google Scholar] [CrossRef]
- Liu, Y.; Koyano, G.; Na, K.; Misono, M. Isomerization of n-pentane and n-hexane over cesium hydrogen salt of 12-tungstophosphoric acid promoted by platinum. Appl. Catal. A Gen. 1998, 166, L263–L265. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Direct oxidation of benzene to phenol by molecular oxygen over catalytic systems containing Pd(OAc)2 and heteropolyacid immobilized on HMS or PIM. J. Mol. Catal. A Chem. 2006, 256, 247–255. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Liquid-phase oxidation of benzene to phenol by molecular oxygen over transition metal substituted polyoxometalate compounds. Catal. Commun. 2005, 6, 679–683. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M.; Mimura, N. Selective oxidation of propylene to acetone by molecular oxygen over Mx/2H5-x[PMo10V2O40]/HMS (M = Cu2+, Co2+, Ni2+). Catal. Commun. 2003, 4, 281–285. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Heterogeneous carbonylation of dimethyl ether to methyl acetate over bifunctional catalysts containing Rh and heteropoly acids. React. Kinet. Mech. Catal. 2016, 117, 223–238. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M.; Nakajima, H.; Koya, M.; Tomokuni, K. Catalytic oxidation of cyclohexene by molecular oxygen over isopolyoxometalates. Chem. Lett. 2004, 33, 200–201. [Google Scholar] [CrossRef]
- Venturello, C.; D’Aloisio, R.; Bart, J.C.; Ricci, M. A new peroxotungsten heteropoly anion with special oxidizing properties: Synthesis and structure of tetrahexylammonium tetra(diperoxotungsto)phosphate(3–). J. Mol. Catal. 1985, 32, 107–110. [Google Scholar] [CrossRef]
- Gao, Y.; Juliao, D.; Silva, D.F.; Castro, B.; Zhao, J.; Balula, S.S. A simple desulfurization process to achieve high efficiency, sustainability and cost-effectivity via peroxotungstate catalyst. Mol. Catal. 2021, 505, 111515. [Google Scholar] [CrossRef]
- Kruse, J.H.; Langer, M.; Romanenko, I.; Trentin, I.; Hernández-Castillo, D.; González, L.; Schacher, F.H.; Streb, C. Polyoxometalate-soft matter composite materials: Design strategies, applications, and future directions. Adv. Funct. Mater. 2022, 2208428. [Google Scholar] [CrossRef]
- Buru, C.T.; Farha, O.K. Strategies for incorporating catalytically active polyoxometalates in metal−organic frameworks for organic transformations. ACS Appl. Mater. Interfaces 2020, 12, 5345–5360. [Google Scholar] [CrossRef]
- Gelbard, G.; Gauducheau, T.; Vidal, E.; Parvulescu, V.I.; Crosman, A.; Pop, V.M. Epoxidation with peroxotungstic acid immobilized onto silica-grafted phosphoramides. J. Mol. Catal. A Chem. 2002, 182–183, 257–266. [Google Scholar] [CrossRef]
- Gelbard, G.; Raison, F.; Roditi-Lachter, E.; Thouvenot, R.; Ouahab, L.; Grandjean, D. Epoxidation of allylic alcohols by hydrogen peroxide in the presence of complexed peroxotungstic species. J. Mol. Catal. A Chem. 1996, 114, 77–85. [Google Scholar] [CrossRef]
- Gao, Y.; Mirante, F.; de Castro, B.; Zhao, J.; Cunha-Silva, L.; Balula, S.S. An effective hybrid heterogeneous catalyst to desulfurize diesel: Peroxotungstate@Metal–organic framework. Molecules 2020, 25, 5494. [Google Scholar] [CrossRef] [PubMed]
- Chilivery, R.; Chaitanya, V.; Nayak, J.; Seth, S.; Rana, R.K. Heterogenization of phosphotungstate clusters into magnetic microspheres: Catalyst for selective oxidation of alcohol in water. ACS Sustain. Chem. Eng. 2022, 10, 6925–6933. [Google Scholar] [CrossRef]
- Leng, Y.; Wu, J.; Jiang, P.; Wang, J. Amphiphilic phosphotungstate-paired ionic copolymer as a highly efficient catalyst for triphase epoxidation of alkenes with H2O2. Catal. Sci. Technol. 2014, 4, 1293–1300. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef] [PubMed]
- Gruselle, M. Apatites: A new family of catalysts in organic synthesis. J. Organomet. Chem. 2015, 793, 93–101. [Google Scholar] [CrossRef]
- Ichihara, J.; Yamaguchi, S.; Nomoto, T.; Nakayama, H.; Iteya, K.; Naitoh, N.; Sasaki, Y. Keggin-type polyacid cluster on apatite: Characteristic catalytic activities in solvent-free oxidation. Tetrahedron Lett. 2002, 43, 8231–8234. [Google Scholar] [CrossRef]
- Ichihara, J.; Kambara, A.; Iteya, K.; Sugimoto, E.; Shinkawa, T.; Takaoka, A.; Yamaguchi, S.; Sasaki, Y. Cetylpyridinium dodecatungstate on fluorapatite: Efficient and reusable solid catalyst for solvent-free epoxidation. Green Chem. 2003, 5, 491–493. [Google Scholar] [CrossRef]
- Crosman, A.; Gelbard, G.; Poncelet, G.; Parvulescu, V.I. Epoxidation of cyclohexene and indene with hydrogen peroxide in the presence of WO5 onto hydroxyapatite as a catalyst. Appl. Catal. A Gen. 2004, 264, 23–32. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J. Mater. Sci.-Mater. Med. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Baev, S.G.; Matvienko, A.A.; Gerasimov, K.B.; Logutenko, O.A.; Bystrov, V.S. A Study of Thermal Stability of Hydroxyapatite. Minerals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Mortier, A.; Lemaitre, J.; Rodrique, L.; Rouxhet, P.G. Synthesis and thermal behavior of well-crystallized calcium-deficient phosphate apatite. J. Solid State Chem. 1989, 78, 215–219. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kanai, H.; Moffat, J.B. Catalytic oxidation of carbon monoxide over stoichiometric and non-stoichiometric hydroxyapatites. J. Chem Soc. Faraday Trans. 1997, 93, 4283–4387. [Google Scholar] [CrossRef]
- Tanaka, H.; Futaoka, M.; Hino, R. Surface modification of calcium hydroxyapatite with pyrophosphoric acid. J. Colloid Interface Sci. 2004, 269, 358–363. [Google Scholar] [CrossRef]
- Mori, K.; Hara, T.; Oshiba, M.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Catalytic investigations of carbon-carbon bond-forming reactions by a hydroxyapatite-bound palladium complex. New J. Chem. 2005, 29, 1174–1181. [Google Scholar] [CrossRef]
- Kaur, J.; Kozhevnikov, I.V. Polyoxometalate-catalysed epoxidation of propylene with hydrogen peroxide: Microemulsion versus biphasic process. Catal. Commun. 2004, 5, 709–713. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Creation of a monomeric Ru species on the surface of hydroxyapatite as an efficient heterogeneous catalyst for aerobic alcohol oxidation. J. Am. Chem. Soc. 2000, 122, 7144–7145. [Google Scholar] [CrossRef]
- Mori, K.; Yamaguchi, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Controlled synthesis of hydroxyapatite-supported palladium complexes as highly efficient heterogeneous catalyst. J. Am. Chem. Soc. 2002, 124, 11572–11573. [Google Scholar] [CrossRef]
- Hara, T.; Mori, K.; Oshiba, M.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Highly efficient dehalogenation using hydroxyapatite-supported palladium nanocluster catalyst with molecular hydrogen. Green Chem. 2004, 6, 507–509. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Chen, J.; Zeng, S.; DE Groot, K. High temperature characteristics of synthetic hydroxyapatite. J. Mater. Sci.-Mater. Med. 1993, 4, 83–85. [Google Scholar] [CrossRef]
- Li, Y.; De Groot, K.; De Wijn, J.; Klein, C.P.A.T.; Meer, S.V.D. Morphology and composition of nanograde calcium phosphate needle-like crystals formed by simple hydrothermal treatment. J. Mater. Sci.-Mater. Med. 1994, 5, 326–331. [Google Scholar]
- Tamai, M.; Nakamura, M.; Isshiki, T.; Nishio, K.; Endoh, H.; Nakahira, A. A metastable phase in thermal decomposition of Ca-deficient hydroxyapatite. J. Mater. Sci.-Mater. Med. 2003, 14, 617–622. [Google Scholar] [CrossRef]
- Tanaka, H.; Cjikazawa, M.; Kandori, K.; Ishikawa, T. FTIR and TPD studies on the adsorption of pyridine, n-butylamine and acetic acid on calcium hydroxyapatite. Phys. Chem. Chem. Phys. 2000, 2, 2647–2650. [Google Scholar] [CrossRef]
- Aubry, C.; Chottard, G.; Platzer, N.; Bregeault, J.M.; Thouvenot, R.; Chauveau, F.; Huet, C.; Ledon, H. Reinvestigation of epoxidation using tungsten-based precursors and hydrogen peroxide in a biphase medium. Inorg. Chem. 1991, 30, 4409–4415. [Google Scholar] [CrossRef]
- Dengel, A.C.; Griffith, W.P.; Parkin, B.C. Studies on polyoxo- and polyperoxo-metalates. Part 1 Tetrameric heteropolyperoxotungstates and heteropolyperoxomolybdates. J. Chem. Soc. Dalton Trans. 1993, 2683–2688. [Google Scholar] [CrossRef]
- Bulina, N.V.; Eremina, N.V.; Vinokurova, O.B.; Ishchenko, A.V.; Chaikina, M.V. Diffusion of copper ions in the lattice of substituted hydroxyapatite during heat treatment. Materials 2022, 15, 5759. [Google Scholar] [CrossRef] [PubMed]
- Bulina, N.V.; Rybin, D.K.; Makarova, S.V.; Dudina, D.V.; Batraev, I.S.; Utkin, A.V.; Prosanov, I.Y.; Khvostov, M.V.; Ulianitsky, V.Y. Detonation Spraying of Hydroxyapatite on a Titanium Alloy Implant. Materials 2021, 14, 4852. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Suzuki, K.; Hamakawa, S.; Tsunoda, T.; Ishii, T.; Kumagai, M. Highly active copper/ceria catalysts for steam reforming of methanol. Appl. Catal. A-Gen. 2002, 223, 137–145. [Google Scholar] [CrossRef]
- Jacob, P.; Wehling, B.; Hill, W.; Klockow, D. Feasibility study of Raman spectroscopy as a tool to investigate the liquid-phase chemistry of aliphatic organic peroxides. Appl. Spectrosc. 1997, 51, 74–80. [Google Scholar] [CrossRef]
- Yeom, Y.H.; Ulagappan, N.; Frei, H. Mechanistic Study of CH3OH + O2 photoredox reaction in a FeAlPO4 sieve by time-resolved FT-IR spectroscopy. J. Phys. Chem. A 2002, 106, 3350–3355. [Google Scholar] [CrossRef]
- Bauerl, S.; Moortgat, G.K. Absorption cross-sections of HOCHOOH vapor between 205 and 360 nm at 298 K. Chem. Phys. Lett. 1999, 309, 43–48. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyas, Y.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of vegetable oils to produce bio-hydrogenated diesel and liquefied petroleum gas fuel over catalysts containing sulfided Ni-Mo and solid acids. Energy Fuels 2011, 25, 4675–4685. [Google Scholar] [CrossRef]











| Compound | Chemical Composition (wt.%) | Molar Ratio of Ca/P | |||
|---|---|---|---|---|---|
| Ca | P | W | Pd | ||
| HAP | 34.3 | 19.8 | 0 | 0 | 1.34 |
| PW-HAP | 26.8 | 15.6 | 13.6 | 0 | 1.33 |
| Pd-PW-HAP | 26.1 | 15.2 | 13.2 | 1.2 | 1.33 |
| Catalyst 2 | C3H6 Conv. (%) | Selectivity (%) | PO Yield (%) | TON of [O–O] 2− | |||||
|---|---|---|---|---|---|---|---|---|---|
| PO | Acrolein | PA 3 | Acetone | C3H8 | Others 4 | ||||
| PW-HAP | 2.3 | 90.3 | 3.4 | 2.5 | 0 | 0 | 3.8 | 2.1 | 1.1 |
| PdCl2(PhCN)2 | 2.1 | 9.5 | 51.6 | 6.4 | 7.3 | 20.7 | 4.5 | 0.2 | – |
| Pd-PW-HAP | 53.4 | 88.7 | 3.6 | 2.3 | 0.8 | 0.9 | 3.7 | 47.4 | 23.6 |
| Pd+PW2 | 57.2 | 88.5 | 3.5 | 2.4 | 0.8 | 1.0 | 3.8 | 50.6 | 25.2 |
| Pd-HMS-PW2 | 44.6 | 89.6 | 2.3 | 1.8 | 1.3 | 1.1 | 3.9 | 40.0 | 19.9 |
| PdMgAl-PW4 | 48.3 | 87.1 | 3.8 | 2.9 | 1.2 | 0.9 | 4.1 | 42.1 | 21.0 |
| Catalyst | Run No. | C3H6 Conv. (%) | Select. for PO (%) | PO Yield (%) |
|---|---|---|---|---|
| Fresh | 1 | 53.4 | 88.7 | 47.3 |
| Used | 2 | 53.4 | 88.8 | 47.4 |
| 3 | 53.3 | 88.7 | 47.3 | |
| 4 | 53.4 | 88.6 | 47.3 | |
| 5 | 53.3 | 88.8 | 47.3 |
| Solvent | C3H6 Conversion (%) | Selectivity for PO (%) | PO Yield (%) | TON of [O–O]2− |
|---|---|---|---|---|
| Methanol | 53.4 | 88.7 | 47.4 | 23.6 |
| Chloroform | 2.2 | 91.5 | 2.0 | 1.0 |
| Catalyst | Amounts of Consumption (mmol) | Amounts of Formation (mmol) | ||||
|---|---|---|---|---|---|---|
| C3H6 (mmol) | O2 (mmol) | CH3OH (mmol) | PO (mmol) | By-Product (mmol) 2 | Co-Product (mmol) 3 | |
| PW-HAP | 0.7 | 0.4 | 0 | 0.6 | 0.1 | 0 |
| Pd-PW-HAP | 18.8 | 20.3 | 10.1 | 17.0 | 1.8 | 10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y. Immobilization of Peroxo-Heteropoly Compound and Palladium on Hydroxyapatite for the Epoxidation of Propylene by Molecular Oxygen in Methanol. Molecules 2023, 28, 24. https://doi.org/10.3390/molecules28010024
Liu Y. Immobilization of Peroxo-Heteropoly Compound and Palladium on Hydroxyapatite for the Epoxidation of Propylene by Molecular Oxygen in Methanol. Molecules. 2023; 28(1):24. https://doi.org/10.3390/molecules28010024
Chicago/Turabian StyleLiu, Yanyong. 2023. "Immobilization of Peroxo-Heteropoly Compound and Palladium on Hydroxyapatite for the Epoxidation of Propylene by Molecular Oxygen in Methanol" Molecules 28, no. 1: 24. https://doi.org/10.3390/molecules28010024
APA StyleLiu, Y. (2023). Immobilization of Peroxo-Heteropoly Compound and Palladium on Hydroxyapatite for the Epoxidation of Propylene by Molecular Oxygen in Methanol. Molecules, 28(1), 24. https://doi.org/10.3390/molecules28010024