Comparative Effects of Two Forms of Chitosan on Selected Phytochemical Properties of Plectranthus amboinicus (Lour.)
Abstract
:1. Introduction
2. Results
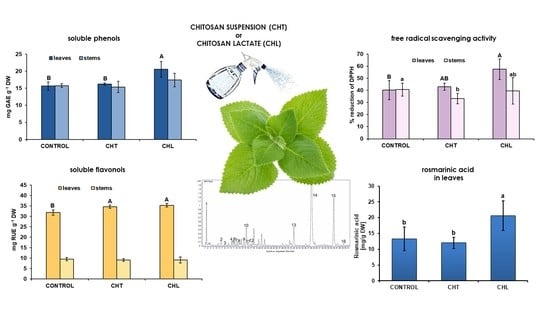
2.1. Content of Polyphenolic Compounds and Antioxidant Activity after Application of Chitosan
2.2. Principle Component Analysis
2.3. Dry Weight of Aboveground Parts and Selected Parameters of Chlorophyll a Fluorescence after Application of Chitosan
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Conditions
4.2. Methods of Plant Material Analysis
4.2.1. Preparation of Methanol Extracts
4.2.2. Determination of Total Soluble Phenolic Compounds
4.2.3. Determination of Total Soluble Flavonols
4.2.4. Determination of Total Soluble Anthocyanins
4.2.5. Determination of Free Radical-Scavenging Activity with the DPPH Method
4.2.6. UHPLC-MS Analysis
4.2.7. Biometric Parameters
4.2.8. Measurement of Selected Parameters of Chlorophyll a Fluorescence
4.3. Statistical Analysisok
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fierascu, R.C.; Fierascu, I.; Baroi, A.M.; Ortan, A. Selected aspects related to medicinal and aromatic plants as alternative sources of bioactive compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef] [PubMed]
- Souri, E.; Amin, G.; Farsam, H.; Jalalizadeh, H.; Barezi, S. Screening of thirteen medicinal plant extracts for antioxidant activity. Iran, J. Pharm. Res. 2022, 7, 128584. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar] [CrossRef]
- Gorelick, J.; Bernstein, N. Elicitation: An underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Adv. Agron. 2014, 124, 201–230. [Google Scholar] [CrossRef]
- Baenas, N.; Garcia-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Arom. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Amborabe, B.-E.; Bonmort, J.; Fleurat-Lessard, P.; Roblin, G. Early events induced by chitosan on plant cells. J. Exp. Bot. 2008, 59, 2317–2324. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, biostimulating, and eliciting effects of chitosan and its derivatives on crop plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef]
- Ferri, M.; Tassoni, A. Chitosan as elicitor of health beneficial secondary metabolites in in vitro plant cell cultures. In Handbook of Chitosan Research and Applications; Mackay, R.G., Tait, J.M., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; pp. 389–414. [Google Scholar]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.-H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujˇci´c Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Froldi, G.; Ragazzi, E. Selected plant-derived polyphenols as potential therapeutic agents for peripheral artery disease: Molecular mechanisms, efficacy and safety. Molecules 2022, 27, 7110. [Google Scholar] [CrossRef]
- Chen, Z.; Farag, M.; Zhong, Z.; Zhang, C.; Yang, Y.; Shengpeng, W.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 176, 113870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, M.A. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, B.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Fernández-Calvet, A.; Euba, B.; Caballero, L.; Díez-Martínez, R.; Menéndez, M.; Ortiz de Solórzano, C.; Leiva, J.; Micol, V.; Barrajón-Catalán, E.; Garmendia, J. Preclinical evaluation of the antimicrobial-immunomodulatory dual action of xenohormetic molecules against Haemophilus influenzae respiratory infection. Biomolecules 2019, 19, 891. [Google Scholar] [CrossRef] [Green Version]
- Suter, S.; Lucock, M. Xenohormesis: Applying evolutionary principles to contemporary health issues. Explor. Res. Hypothesis Med. 2017, 2, 79–85. [Google Scholar] [CrossRef]
- Kaliappan, N.D.; Viswanathan, P.K. Pharmacognostical studies on the leaves of Plectranthus amboinicus (Lour) Spreng. Int. J. Green Pharm. 2008, 2, 182–184. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Erny Sabrina, M.N.; Razali, M.; Mirfat, A.H.S.; Mohd Shukri, M.A. Antimicrobial activity and bioactive evaluation of Plectranthus amboinicus essential oil. Am. J. Res. Commun. 2014, 2, 121–127. [Google Scholar]
- Aguiar, J.J.S.; Sousa, C.P.B.; Araruna, M.K.A.; Silva, M.K.N.; Portelo, A.C.; Lopes, J.C.; Carvalho, V.R.A.; Figueredo, F.G.; Bitu, V.C.N.; Coutinho, H.D.M.; et al. Antibacterial and modifying-antibiotic activities of the essential oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015, 7, 151–156. [Google Scholar] [CrossRef]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, phytochemical, pharmacological and nutritional significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef] [Green Version]
- Wadikar, D.D.; Patki, P.E. Coleus aromaticus: A therapeutic herb with multiple potentials. J. Food Sci. Technol. 2016, 53, 2895–2901. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.D.; Mahobia, N.K.; Singh, M.P.; Singh, A.; Sheikh, N.W.; Alam, G.; Singh, S.K. Antioxidant potential of leaves of Plectranthus amboinicus (Lour) Spreng. Der Pharm. Lett. 2010, 2, 240–245. [Google Scholar]
- Chiu, Y.J.; Huang, T.H.; Chiu, C.S.; Lu, T.C.; Chen, Y.W.; Peng, W.H.; Chen, C.Y. Analgesic and antiinflammatory activities of the aqueous extract from Plectranthus amboinicus (Lour.) Spreng. Both in vitro and in vivo. Evid. Based Complement. Altern. Med. 2012, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, P.; Majumder, P. Investigation of phytochemicals and anti-convulsant activity of the plant Coleus amboinicus (lour.). Int. J. Green Pharm. 2013, 7, 211–215. [Google Scholar] [CrossRef]
- Lalthazuali; Mathew, N. Mosquito repellent activity of volatile oils from selected aromatic plants. Parasitol Res. 2017, 116, 821–825. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF-MS/MS and UHPLC-MS/MS. J. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, S.; Cieślak, A.; Yanza, Y.R.; Szumacher-Strabel, M.; Varadyova, Z.; Stafiniak, M.; Wojnicz, D.; Matkowski, A. Phytochemical profile and antioxidant activities of Coleus amboinicus Lour. cultivated in Indonesia and Poland. Molecules 2021, 26, 2915. [Google Scholar] [CrossRef] [PubMed]
- Formato, M.; Piccolella, S.; Zidorn, C.; Pacifico, S. UHPLC-HRMS analysis of Fagus sylvatica (Fagaceae) leaves: A renewable source of antioxidant polyphenols. Antioxidants 2021, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Ye, J.; Li, H.; Dong, H.; Xie, N.; Mi, N.; Zhang, Z.; Zou, J.; Jin, H.; Zhang, W. Hybrid multidimensional data acquisition and data processing strategy for comprehensive characterization of known, unknown and isomeric compounds from the compound Dan Zhi Tablet by UPLC-TWIMS-QTOFMS. RSC Adv. 2019, 9, 8714–8727. [Google Scholar] [CrossRef] [Green Version]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/chitosan and its derivatives: Fundamental problems and practical approaches. Biochemistry (Moscow) 2020, 85, S154–S176. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R. Eliciting effect of foliar application of chitosan lactate on the phytochemical properties of Ocimum basilicum L. and Melissa officinalis L. Food Chem. 2021, 342, 128358. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Chitosan as soil amendment affects lettuce growth, photochemical efficiency, and gas exchange. HortTechnology 2018, 28, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.M.; Prithiviraj, B.; Smith, D.L. Effect of foliar application of chitin and chitosan oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 2002, 40, 621–624. [Google Scholar] [CrossRef]
- Van, S.N.; Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar] [CrossRef]
- Yin, H.; Frette, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Fooladi Vanda, G.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, J.; Gai, Q.-Y.; Wang, X.; Qin, Q.-P.; Wang, Z.-Y.; Liu, J.; Fu, Y.-J. Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crop. Prod. 2018, 124, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crop. Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Salimgandomi, S.; Shabrangy, A. The effect of chitosan on antioxidant activity and some secondary metabolites of Mentha piperita L. J. Pharm. Health Sci. 2016, 4, 135–142. [Google Scholar]
- Ghasemnezhad, M.; Shiri, M.A.; Sanavi, M. Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Casp. J. Environ. Sci. 2010, 8, 25–33. [Google Scholar]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2019, 19, 2194. [Google Scholar] [CrossRef] [Green Version]
- Badawy, M.E.I.; Rabea, E.I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Boland, A.; Cambier, P.; Frettinger, P.; Van Cutsem, P. Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 2010, 20, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Farooq, T.; Akram, M.N.; Hameed, A.; Ahmed, T.; Hameed, A. Nanopriming-mediated memory imprints reduce salt toxicity in wheat seedlings by modulating physiobiochemical attributes. BMC Plant Biol. 2022, 22, 540. [Google Scholar] [CrossRef]
- Kim, H.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Poterańska, N.; Mijowska, K.; Ochmian, I. The influence of foliar calcium fertilizers and bio-stimulators on bushes growth, yield and fruit quality of blue honeysuckle (Lonicera caerulea L.) Czarna cultivar. In Research and Development of Young Scientists in Poland–Life Sciences; Young Scientists: Szczecin, Poland, 2015; pp. 132–138. (In Polish) [Google Scholar]
- El-Miniawy, S.M.; Ragab, M.E.; Youssef, S.M.; Metwally, A.A. Response of strawberry plants to foliar spraying of chitosan. Res. J. Agric. Biol. Sci. 2013, 9, 366–372. [Google Scholar]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012, 2012, 104565. [Google Scholar] [CrossRef]
- Mondal, M.; Malek, M.; Puteh, A.; Ismail, M.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop. Sci. 2012, 6, 918–921. [Google Scholar]
- Sathiyanarayanan, A.; Sathiyabama, M. Effect of chitosan on growth, yield and curcumin content in turmeric under field condition. Biocatal. Agric. Biotechnol. 2016, 6, 102–106. [Google Scholar] [CrossRef]
- Safikhan, S.; Khoshbakht, K.; Chaichi, M.R.; Amini, A.; Motesharezadeh, B. Role of chitosan on the growth, physiological parameters and enzymatic activity of milk thistle (Silybum marianum (L.) Gaertn.) in a pot experiment. J. Appl. Res. Med. Aromat. Plants 2018, 10, 49–58. [Google Scholar] [CrossRef]
- Mathew, R.; Sankar, P.D. Effect of methyl jasmonate and chitosan on growth characteristics of Ocimum basilicum L., Ocimum sanctum L. and Ocimum gratissimum L. cell suspension cultures. Afr. J. Biotechnol. 2012, 11, 4759–4766. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhang, S.; Wang, P.; Hou, J.; Qian, J. Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natans. Ecotoxicol. Environ. Saf. 2011, 74, 1297–1303. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia V; Polish Pharmaceutical Society: Warsaw, Poland, 1999; pp. 56–57. (In Polish)
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F.1.2., 1–13. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]





| Peak | RT [min] | [M − H]−(Fragments) | Δppm | Formula | Identified | References |
|---|---|---|---|---|---|---|
| 1 | 1.54 | 191.05648 | 1.92 | C7H12O6 | Quinic acid | Standard |
| 2 | 6.64 | 315.07341 (153) | 3.97 | C13H16O9 | Dihydroxybenzoic acid hexoside | [32] |
| 3 | 7.89 | 153.01942 | 0.57 | C7H6O4 | Dihydroxybenzoic acid | [33] |
| 4 | 10.28 | 353.08901 (191, 179, 135) | 3.40 | C16H18O9 | Neochlorogenic acid | Standard |
| 5 | 10.99 | 341.08831 (179, 135, 221) | 1.47 | C15H18O9 | Caffeoylglucose I | [33] |
| 6 | 11.32 | 297.06176 (179, 135, 117) | 0.59 | C13H14O8 | Caffeic acid derivative I | [34] |
| 7 | 12.37 | 297.06282 (179, 135, 117) | 4.12 | C13H14O8 | Caffeic acid derivative II | [34] |
| 8 | 13.00 | 341.08791 (179, 135, 221) | 0.30 | C15H18O9 | Caffeoylglucose II | [33] |
| 9 | 14.60 | 341.08911 (179, 135, 221) | 3.81 | C15H18O9 | Caffeoylglucose III | [33] |
| 10 | 15.43 | 353.08921 (191, 179, 135) | 3.97 | C16H18O9 | Chlorogenic acid | Standard |
| 11 | 16.05 | 179.03556 | 3.21 | C9H8O4 | Caffeic acid | Standard |
| 12 | 17.66 | 297.06259 (179, 135, 117) | 3.35 | C13H14O8 | Caffeic acid derivative III | [34] |
| 13 | 32.49 | 521.13221 (359, 341, 179) | 4.11 | C24H26O13 | Rosmarinic acid glucoside | [33] |
| 14 | 38.52 | 359.07791 (179, 161) | 1.86 | C18H16O8 | Rosmarinic acid | Standard [33] |
| 15 | 46.41 | 717.14781 (519, 321, 339) | 2.37 | C36H30O16 | Salvianolic acid B | [33,35] |
| 16 | 49.43 | 503.08523 (285, 443) | 4.20 | C23H20O13 | Luteolin 30-(4″-acetylglucuronide) | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasińska-Jakubas, M.; Hawrylak-Nowak, B.; Wójciak, M.; Dresler, S. Comparative Effects of Two Forms of Chitosan on Selected Phytochemical Properties of Plectranthus amboinicus (Lour.). Molecules 2023, 28, 376. https://doi.org/10.3390/molecules28010376
Stasińska-Jakubas M, Hawrylak-Nowak B, Wójciak M, Dresler S. Comparative Effects of Two Forms of Chitosan on Selected Phytochemical Properties of Plectranthus amboinicus (Lour.). Molecules. 2023; 28(1):376. https://doi.org/10.3390/molecules28010376
Chicago/Turabian StyleStasińska-Jakubas, Maria, Barbara Hawrylak-Nowak, Magdalena Wójciak, and Sławomir Dresler. 2023. "Comparative Effects of Two Forms of Chitosan on Selected Phytochemical Properties of Plectranthus amboinicus (Lour.)" Molecules 28, no. 1: 376. https://doi.org/10.3390/molecules28010376
APA StyleStasińska-Jakubas, M., Hawrylak-Nowak, B., Wójciak, M., & Dresler, S. (2023). Comparative Effects of Two Forms of Chitosan on Selected Phytochemical Properties of Plectranthus amboinicus (Lour.). Molecules, 28(1), 376. https://doi.org/10.3390/molecules28010376