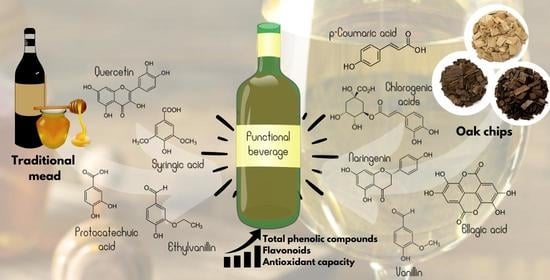
Enhancement of the Functional Properties of Mead Aged with Oak (Quercus) Chips at Different Toasting Levels
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Analytical Reagents
3.2. Experimental Design
3.3. Samples Acquirement
3.4. Total Phenolic Content
3.5. Flavonoids Content
3.6. Antioxidant Capacity Determination
3.7. Purification Procedure in SPE C18
3.8. Phenolic Compound Identification by LC-ESI-QTOF-MS/MS
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sethi, G.; Vidyalaya, K.M.; Raina, A.; Vidyalaya, K.M. Consumers’ Awareness about Nutritional Aspects of Healthy Food: A Qualitative Study. Universe Int. J. Interdiscip. Res. 2020, 2020, 438–443. [Google Scholar]
- Zaidel, D.N.A.; Muhamad, I.I.; Hashim, Z.; Jusoh, Y.M.M.; Salleh, E. Innovation and Challenges in the Development of Functional and Medicinal Beverages. In Functional Foods and Nutraceuticals for Human Health; Apple Academic Press: Boca Raton, FL, USA, 2021; pp. 137–198. [Google Scholar]
- Cong, L.; Bremer, P.; Mirosa, M. Functional Beverages in Selected Countries of Asia Pacific Region: A Review. Beverages 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Radonjić, S.; Maraš, V.; Raičević, J.; Košmerl, T. Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules 2020, 25, 4960. [Google Scholar] [CrossRef] [PubMed]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef]
- Farah, A.; dePaula Lima, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Kahoun, D.; Řezková, S.; Královský, J. Effect of Heat Treatment and Storage Conditions on Mead Composition. Food Chem. 2017, 219, 357–363. [Google Scholar] [CrossRef]
- Starowicz, M.; Granvogl, M. Trends in Food Science & Technology an Overview of Mead Production and the Physicochemical, Toxicological, and Sensory Characteristics of Mead with a Special Emphasis on Flavor. Trends Food Sci. Technol. 2020, 106, 402–416. [Google Scholar] [CrossRef]
- Akalın, H.; Bayram, M.; Anlı, R.E. Determination of Some Individual Phenolic Compounds and Antioxidant Capacity of Mead Produced from Different Types of Honey. J. Inst. Brew. 2017, 123, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, M.; Szwengiel, A. Distinguishing between Saturated and Unsaturated Meads Based on Their Chemical Characteristics. LWT 2020, 133, 109962. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Švecová, B.; Bordovská, M.; Kalvachová, D.; Hájek, T. Analysis of Czech Meads: Sugar Content, Organic Acids Content and Selected Phenolic Compounds Content. J. Food Compos. Anal. 2015, 38, 80–88. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Garde-Cerdán, T.; Martínez, J. Use of Oak Fragments during the Aging of Red Wines. Effect on the Phenolic, Aromatic, and Sensory Composition of Wines as a Function of the Contact Time with the Wood. Beverages 2018, 4, 102. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gil, A.M.; del Alamo-Sanza, M.; del Barrio-Galán, R.; Nevares, I. Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review. Appl. Sci. 2022, 12, 2101. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; del Álamo, M.; Nevares, I. Effect of Size, Seasoning and Toasting in the Volatile Compounds in Toasted Oak Wood and in a Red Wine Treated with Them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef]
- Martínez, J.; Cadahía, E.; Fernández de Simón, B.; Ojeda, S.; Rubio, P. Effect of the Seasoning Method on the Chemical Composition of Oak Heartwood to Cooperage. J. Agric. Food Chem. 2008, 56, 3089–3096. [Google Scholar] [CrossRef]
- Farrell, R.R.; Wellinger, M.; Gloess, A.N.; Nichols, D.S.; Breadmore, M.C.; Shellie, R.A.; Yeretzian, C. Real-Time Mass Spectrometry Monitoring of Oak Wood Toasting: Elucidating Aroma Development Relevant to Oak-Aged Wine Quality. Sci. Rep. 2015, 5, 17334. [Google Scholar] [CrossRef] [Green Version]
- Fernández de Simón, B.; Muiño, I.; Cadahía, E. Characterization of Volatile Constituents in Commercial Oak Wood Chips. J. Agric. Food Chem. 2010, 58, 9587–9596. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Anjos, O.; Belchior, A.P. Phenolic Profile and Colour Acquired by the Wine Spirit in the Beginning of Ageing: Alternative Technology Using Micro-Oxygenation vs Traditional Technology. LWT 2019, 111, 260–269. [Google Scholar] [CrossRef]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in Contact with Oak Wood: The Impact of the Variety (Carménère and Cabernet Sauvignon), Format (Barrels, Chips and Staves), and Aging Time on the Phenolic Composition. J. Sci. Food Agric. 2019, 99, 436–448. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and Validation of a LC-ESI-MS/MS Method for the Determination of Phenolic Compounds in Honeydew Honeys with the Diluted-and-Shoot Approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS Characterization of Phenolic Compounds in Palm Fruits (Jelly and Fishtail Palm) and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Zhu, J.; Zhao, Q.; Hardie, J.; Hu, B. Changes in the Profile of Aroma Compounds in Vitis vinifera L. Cv Merlot from Grapes to Wine. Bangladesh J. Bot. 2017, 46, 1089–1098. [Google Scholar]
- Simonelt, B.R.T.; Rogge, W.F.; Mazurek, M.A.; Standley, L.J.; Hildemann, L.M.; Cass, G.R. Lignin Pyrolysis Products, Lignans, and Resin Acids as Specific Tracers of Plant Classes in Emissions from Biomass Combustion. Environ. Sci. Technol. 1993, 27, 2533–2541. [Google Scholar] [CrossRef]
- Abdulsallam, B.; Ohood Hasan, A.; Nael Abu, T.; Ali, A.-R.; Sachil, K. Chemical Composition of Propolis from the Baha Region in Saudi Arabia. Czech J. Food Sci. 2018, 36, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Maia, I.R.D.O.; Trevisan, M.T.S.; Silva, M.G.D.V.; Breuer, A.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Senna macranthera Var Pudibunda from the Northeast of Brazil. Nat. Prod. Commun. 2019, 14, 1934578X1985170. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Czyżewska, U.; Jankowska, E.; Bakier, S. Determination of Royal Jelly Acids in Honey. Food Chem. 2011, 124, 387–391. [Google Scholar] [CrossRef]
- Lucas, C.I.S.; Ferreira, A.F.; Costa, M.A.P.D.C.; Silva, F.D.L.; Estevinho, L.M.; Carvalho, C.A.L.D. Phytochemical Study and Antioxidant Activity of Dalbergia ecastaphyllum. Rodriguésia 2020, 71, 1–15. [Google Scholar] [CrossRef]
- Le Floch, A.; Jourdes, M.; Teissedre, P.-L. Polysaccharides and Lignin from Oak Wood Used in Cooperage: Composition, Interest, Assays: A Review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef]
- Mosedale, J.; Puech, J.-L. Wood Maturation of Distilled Beverages. Trends Food Sci. Technol. 1998, 9, 95–101. [Google Scholar] [CrossRef]
- Niemelä, O.; Aalto, M.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Laatikainen, T. Alcohol Drinking Patterns and Laboratory Indices of Health: Does Type of Alcohol Preferred Make a Difference? Nutrients 2022, 14, 4529. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, Y.-C.; Chen, S.-J.; Lee, C.-H.; Cheng, C.-M. Alcohol Addiction, Gut Microbiota, and Alcoholism Treatment: A Review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Wu, J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, F1.1.1–F1.1.11. [Google Scholar] [CrossRef]
- Bochi, V.C.; Barcia, M.T.; Rodrigues, D.; Speroni, C.S.; Giusti, M.M.; Godoy, H.T. Polyphenol Extraction Optimisation from Ceylon Gooseberry (Dovyalis hebecarpa) Pulp. Food Chem. 2014, 164, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Quatrin, A.; Pauletto, R.; Maurer, L.; Minuzzi, N.; Nichelle, S.; Carvalho, J.; Maróstica Junior, M.; Rodrigues, E.; Bochi, V.; Emanuelli, T. Characterization and Quantification of Tannins, Flavonols, Anthocyanins and Matrix-Bound Polyphenols from Jaboticaba Fruit Peel: A Comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]



| Tentative Indentification | RT (min) | Molecular Formula | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Fragmentation Ion (m/z) | BM | MOWT | MOMT | MOHT | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Citric acid | 2.1 | C6H8O7 | 192.0270 | 191.0197 | 191.0368 | 111.0205 | X | X | X | X | [12] |
| 3-Hydroxy-3-(3-hydroxyphenyl) propionic acid | 9.0 | C9H10O4 | 182.0579 | 181.0506 | 181.0533 | 121.0420/122.0480 | X | X | [23] | ||
| 2,3-Dihydroxy-1-guaiacylpropanone | 9.2 | C10H12O5 | 212.0685 | 211.0612 | 211.0789 | 134.0499/150.0450 | X | [23] | |||
| Chlorogenic acid | 9.2 | C16H18O9 | 354.0951 | 353.0878 | 353.0899 | 191.0574 | X | X | [22] | ||
| Protocatechuic acid | 9.3 | C7H6O4 | 154.0266 | 153.0193 | 153.0335 | 109.0376 | X | X | X | X | [12] |
| Butanedioic acid | 10.6 | C8H14O5 | 190.0841 | 189.0768 | 189.0791 | 129.0680/127.0878/99.0934 | X | X | [24] | ||
| Vanillin | 10.7 | C8H8O3 | 152.0475 | 151.0403 | 151.0414 | 108.0181 | X | X | X | [12] | |
| Sinapyl alcohol | 10.7 | C11H14O4 | 210.0892 | 209.0819 | 209.0996 | 137.0266 | X | X | X | X | [25] |
| Syringic acid | 10.8 | C9H10O5 | 198.0528 | 197.0455 | 197.0477 | 111.0182/125.0360/140.0247 | X | X | X | X | [22] |
| Ethylvanillin | 11.0 | C9H10O3 | 166.0630 | 165.0557 | 165.0583 | 119.0514/117.0355 | X | X | X | X | [12] |
| p-Coumaric | 11.1 | C9H8O3 | 164.0473 | 163.0401 | 163.0401 | 119.0518 | X | X | X | [10] | |
| 1-(2-hydroxy-4,6-dimethoxyphenyl)-ethanone | 11.4 | C10H12O4 | 196.0736 | 195.0663 | 195.0690 | 117.0337/134.0387 | X | X | X | X | [26] |
| Ellagic acid | 11.6 | C14H6O8 | 302.0063 | 300.9990 | 301.0017 | 229.0170/301.0018 | X | X | X | [27] | |
| Abscísic acid | 17.1 | C15H20O4 | 264.1362 | 263.1289 | 263.1313 | 136.0543/203.1091 | X | X | X | [22] | |
| Sebacic acid | 17.5 | C10H18O4 | 202.1205 | 201.1132 | 201.1302 | 183.1170/139.1259 | X | X | X | X | [28] |
| Quercetin | 17.7 | C15H12O5 | 302.0427 | 301.0354 | 301.0584 | 151.0175/107.0253/116.0828/121.0426 | X | X | X | X | [22] |
| Naringenin | 18.5 | C15H10O7 | 272.0685 | 271.0612 | 271.0632 | 125.0266/197.0639/225.0540/253.0480 | X | X | X | [10] | |
| Tiliroside | 20.7 | C30H26O13 | 594.1373 | 593.1301 | 593.1326 | 121.0298/209.0480/417.0965 | X | X | [29] |
| Code | Treatment |
|---|---|
| BM | Base mead—not aged with oak chips |
| MOWT | Mead aged with oak chips without toasting |
| MOMT | Mead aged with oak chips at medium toasting (170 °C for 35 min) |
| MOHT | Mead aged with oak chips at high toasting (200 °C for 45 min) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortes, J.P.; Franco, F.W.; Baranzelli, J.; Ugalde, G.A.; Ballus, C.A.; Rodrigues, E.; Mazutti, M.A.; Somacal, S.; Sautter, C.K. Enhancement of the Functional Properties of Mead Aged with Oak (Quercus) Chips at Different Toasting Levels. Molecules 2023, 28, 56. https://doi.org/10.3390/molecules28010056
Fortes JP, Franco FW, Baranzelli J, Ugalde GA, Ballus CA, Rodrigues E, Mazutti MA, Somacal S, Sautter CK. Enhancement of the Functional Properties of Mead Aged with Oak (Quercus) Chips at Different Toasting Levels. Molecules. 2023; 28(1):56. https://doi.org/10.3390/molecules28010056
Chicago/Turabian StyleFortes, Juciane Prois, Fernanda Wouters Franco, Julia Baranzelli, Gustavo Andrade Ugalde, Cristiano Augusto Ballus, Eliseu Rodrigues, Márcio Antônio Mazutti, Sabrina Somacal, and Claudia Kaehler Sautter. 2023. "Enhancement of the Functional Properties of Mead Aged with Oak (Quercus) Chips at Different Toasting Levels" Molecules 28, no. 1: 56. https://doi.org/10.3390/molecules28010056
APA StyleFortes, J. P., Franco, F. W., Baranzelli, J., Ugalde, G. A., Ballus, C. A., Rodrigues, E., Mazutti, M. A., Somacal, S., & Sautter, C. K. (2023). Enhancement of the Functional Properties of Mead Aged with Oak (Quercus) Chips at Different Toasting Levels. Molecules, 28(1), 56. https://doi.org/10.3390/molecules28010056