Ultrasonication-Tailored Graphene Oxide of Varying Sizes in Multiple-Equilibrium-Route-Enhanced Adsorption for Aqueous Removal of Acridine Orange
Abstract
:1. Introduction
2. Results
2.1. Sonicated Graphene Oxide and Acridine Orange
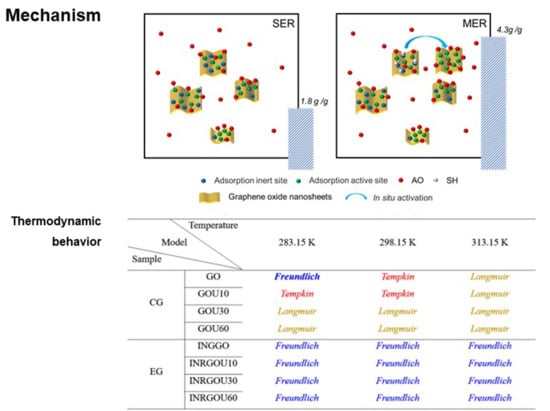
2.2. Comparative Adsorption of the MER and SER Involving Size-Different GO
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of GO
4.3. Preparation of GO and INRGO with Different Sizes
4.4. Adsorption Procedure
4.5. Kinetics Experiment
4.6. Isotherm Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, L.; Leung, M.Y.; Boriskina, S.; Tao, X. Advancing life cycle sustainability of textiles through technological innovations. Nat. Sustain. 2022, 6, 243–253. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, C.; Zhou, W.; Lei, A.; Zhang, Q.; Wan, Q.; Zou, B. Fast and considerable adsorption of methylene blue dye onto graphene oxide. Bull. Env. Contam. Toxicol. 2011, 87, 86–90. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, S.; Yang, L.; Chu, H.; Peng, B.Y.; Xiao, S.; Wang, Y.; Zhou, X.; Zhang, Y. Microalgal wastewater recycling: Suitability of harvesting methods and influence on growth mechanisms. Sci. Total Environ. 2022, 859, 160237. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M. Wastewater Treatment by Adsorption and/or Ion-Exchange Processes for Resource Recovery. Water 2022, 14, 911. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Xing, Z.; Ma, T.; Shi, Z.; Ji, X.; Ma, S. Creation of a new type of ion exchange material for rapid, high-capacity, reversible and selective ion exchange without swelling and entrainment. Chem. Sci. 2016, 7, 2138–2144. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zheng, Z.; Liu, H.; Zhu, L.; Yang, M.; Chen, Y. Nanocellulose-based membranes for highly efficient molecular separation. Chem. Eng. J. 2023, 451, 138711. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Israilides, C.J. Electrochemical oxidation of a textile dye and finishing wastewater using a Pt/Ti electrode. J. Environ. Sci. Health Part A 1998, 33, 847–862. [Google Scholar] [CrossRef]
- Pearce, C. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Kumara, A.V.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)(3)-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- Akrami, M.; Danesh, S.; Eftekhari, M. Comparative Study on the Removal of Cationic Dyes Using Different Graphene Oxide Forms. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1785–1797. [Google Scholar] [CrossRef]
- Qiu, J.H.; Feng, Y.; Zhang, X.F.; Jia, M.M.; Yao, J.F. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef]
- Wang, J.M.; Huang, C.P.; Allen, H.E.; Cha, D.K.; Kim, D.W. Adsorption characteristics of dye onto sludge particulates. J. Colloid Interface Sci. 1998, 208, 518–528. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.-O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Kumar, R.T.R. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and pi-pi interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar]
- Senthamarai, C.; Kumar, P.S.; Priyadharshini, M.; Vijayalakshmi, P.; Kumar, V.V.; Baskaralingam, P.; Thiruvengadaravi, K.V.; Sivanesan, S. Adsorption behavior of methylene blue dye onto surface modified Strychnos potatorum seeds. Environ. Prog. Sustain. Energy 2013, 32, 624–632. [Google Scholar] [CrossRef]
- Zeng, H.X.; Tang, R.C. Adsorption properties of direct dyes on viscose/chitin bicomponent fiber: Evaluation and comparison with viscose fiber. RSC Adv. 2014, 4, 38064–38072. [Google Scholar] [CrossRef]
- Li, Y.; Pan, B.; Miao, H.Y.; Xu, H.M.; Liu, X.F.; Shi, G. Single and Binary Dye Adsorption of Methylene Blue and Methyl Orange in Alcohol Aqueous Solution via Rice Husk Based Activated Carbon: Kinetics and Equilibrium Studies. Chem. Res. Chin. Univ. 2020, 36, 1272–1278. [Google Scholar] [CrossRef]
- Fiallos, D.C.; Gómez, C.V.; Usca, G.T.; Pérez, D.C.; Tavolaro, P.; Martino, G.; Caputi, L.S.; Tavolaro, A. Removal of acridine orange from water by graphene oxide. AIP Conf. Proc 2015, 1646, 38–45. [Google Scholar]
- Gong, J.; Gao, X.; Li, M.; Nie, Q.; Pan, W.; Liu, R. Dye adsorption on electrochemical exfoliated graphene oxide nanosheets: pH influence, kinetics and equilibrium in aqueous solution. Int. J. Environ. Sci. Technol. 2017, 14, 305–314. [Google Scholar] [CrossRef]
- Poh, H.L.; Sanek, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Jirickova, A.; Jankovsky, O.; Sofer, Z.; Sedmidubsky, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Mercier, G.; Klechikov, A.; Hedenström, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Moons, E. Brodie vs. Hummers graphite oxides for preparation of multi-layered materials. Carbon 2017, 115, 430–440. [Google Scholar] [CrossRef]
- Bezerra de Araujo, C.M.; Filipe Oliveira do Nascimento, G.; Rodrigues Bezerra da Costa, G.; Santos da Silva, K.; Salgueiro Baptisttella, A.M.; Gomes Ghislandi, M.; Alves da Motta Sobrinho, M. Adsorptive removal of dye from real textile wastewater using graphene oxide produced via modifications of hummers method. Chem. Eng. Commun. 2018, 206, 1375–1387. [Google Scholar] [CrossRef]
- Muhamad, K.; Mohamed, F.; Radiman, S.; Hamzah, A.; Sarmani, S.; Siong, K.K.; Yasir, M.S.; Rahman, I.A.; Rosli, N. Synthesis and Characterization of Exfoliated Graphene Oxide. In Proceedings of the UKM FST Postgraduate Colloquium, Universiti Kebangsaan Malaysia, Faculty of Science and Technology, Selangor, Malaysia, 13–14 April 2016. [Google Scholar]
- Lu, L.; Wang, J.; Chen, B. Adsorption and desorption of phthalic acid esters on graphene oxide and reduced graphene oxide as affected by humic acid. Env. Pollut. 2018, 232, 505–513. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H.; Fugetsu, B. Graphene oxide adsorption enhanced by in situ reduction with sodium hydrosulfite to remove acridine orange from aqueous solution. J Hazard. Mater. 2012, 203–204, 101–110. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Graphene Oxide for Elimination of Dyes. In Design of Materials and Technologies for Environmental Remediation, 1st ed.; Shunitz, T., Masaaki, K., Masaaki, M., Yuichi, K., Eds.; Springer Nature: Singapore, 2022; Volume 115, pp. 393–422. [Google Scholar]
- Yu, Y.; Wang, Z.; Sun, R.; Chen, Z.; Liu, M.; Zhou, X.; Yao, M.; Wang, G. Self-Supported Reduced Graphene Oxide Membrane and Its Cu(2+) Adsorption Capability. Materials 2020, 14, 146. [Google Scholar] [CrossRef]
- Hao, J.; Ji, L.; Li, C.; Hu, C.; Wu, K. Rapid, efficient and economic removal of organic dyes and heavy metals from wastewater by zinc-induced in-situ reduction and precipitation of graphene oxide. J. Taiwan Inst. Chem. Eng. 2018, 88, 137–145. [Google Scholar] [CrossRef]
- Wernke, G.; Shimabuku-Biadola, Q.L.; Dos Santos, T.R.T.; Silva, M.F.; Fagundes-Klen, M.R.; Bergamasco, R. Adsorption of cephalexin in aqueous media by graphene oxide: Kinetics, isotherm, and thermodynamics. Environ. Sci. Pollut. Res. Int. 2020, 27, 4725–4736. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Xu, X.; Alsaedi, A.; Hayat, T.; Li, J. Adsorption and desorption of U(VI) on different-size graphene oxide. Chem. Eng. J. 2019, 360, 941–950. [Google Scholar] [CrossRef]
- Guo, L.; Liu, L.; Zhuo, M.; Fu, S.; Xu, Y.; Zhou, W.; Shi, C.; Ye, B.; Li, Y.; Chen, W. Smaller-lateral-size graphene oxide hydrosols sealed in dialysis bags for enhanced trace Pb(II) removal from water without re-pollution. Appl. Surf. Sci. 2018, 445, 586–595. [Google Scholar] [CrossRef]
- Huang, J.; Cui, C.; Zhao, X.; Chen, G. Size separation of graphene oxide via multi-layer filtering by silica gel column. Mater. Express 2019, 9, 1025–1032. [Google Scholar] [CrossRef]
- Kang, D.; Shin, H.S. Control of size and physical properties of graphene oxide by changing the oxidation temperature. Carbon Lett. 2012, 13, 39–43. [Google Scholar] [CrossRef]
- Cai, C.; Sang, N.; Shen, Z.; Zhao, X. Facile and size-controllable preparation of graphene oxide nanosheets using high shear method and ultrasonic method. J. Exp. Nanosci. 2017, 12, 247–262. [Google Scholar] [CrossRef]
- Mendez-Romero, U.A.; Alfonso Perez-Garcia, S.; Fan, Q.; Wang, E.; Licea-Jimenez, L. Lateral size reduction of graphene oxide preserving its electronic properties and chemical functionality. RSC Adv. 2020, 10, 29432–29440. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Sun, J.; Wang, J.; Qin, C.; Dai, L. Size fractionation of graphene oxide sheets assisted by circular flow and their graphene aerogels with size-dependent adsorption. RSC Adv. 2016, 6, 74053–74060. [Google Scholar] [CrossRef]
- Wang, X.; Bai, H.; Shi, G. Size fractionation of graphene oxide sheets by pH-assisted selective sedimentation. J. Am. Chem. Soc. 2011, 133, 6338–6342. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Jia, N.; Li, C.; Shi, G. Size Fractionation of Graphene Oxide Sheets via Filtration through Track-Etched Membranes. Adv. Mater. 2015, 27, 3654–3660. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, X.; Chen, B. Size effects of graphene oxide nanosheets on the construction of three-dimensional graphene-based macrostructures as adsorbents. J. Mater. Chem. A 2016, 4, 12106–12118. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, L.; Li, D.Y.; Peng, B.Q.; Shi, G.S.; Xu, G.; Fang, H.P.; Wu, M.-H. Preparation of graphene oxides with different sheet sizes by temperature control. Chin. Phys. B 2017, 26, 106101. [Google Scholar] [CrossRef]
- Deemer, E.M.; Paul, P.K.; Manciu, F.S.; Botez, C.E.; Hodges, D.R.; Landis, Z.; Akter, T.; Castro, E.; Chianelli, R.R. Consequence of oxidation method on graphene oxide produced with different size graphite precursors. Mater. Sci. Eng. B 2017, 224, 150–157. [Google Scholar] [CrossRef]
- Wang, C.C.; Ge, H.Y.; Liu, H.S.; Liang, J.J. Properties of carbon fiber and composites modified with different-sized graphene oxide sheets. Polym. Compos. 2016, 37, 2719–2726. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, T.; Deng, S.; Zong, G.; Yao, X.; Fu, Q. Size-specified graphene oxide sheets: Ultrasonication assisted preparation and characterization. J. Mater Sci. 2013, 49, 1785–1793. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental Remediation Applications of Carbon Nanotubes and Graphene Oxide: Adsorption and Catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, T.; Zhang, H. Molecular Dynamics Simulation of Adsorption of Methylene Blue by Graphene Oxide. Chem. J. Chin. Univ.-Chin. 2019, 40, 2534–2541. [Google Scholar]
- Moon, H.S.; Lee, J.H.; Kwon, S.; Kim, I.T.; Lee, S.G. Mechanisms of Na adsorption on graphene and graphene oxide: Density functional theory approach. Carbon Lett. 2015, 16, 116–120. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, L.B.; Li, R.J.; Xiang, J.Z.; Huang, Q.; Lau, S.P. Graphene oxide: Progress in preparation, reduction and application. J. Infrared Millim. Waves 2019, 38, 79–90. [Google Scholar]
- Feicht, P.; Biskupek, J.; Gorelik, T.E.; Renner, J.; Halbig, C.E.; Maranska, M.; Puchtler, F.; Kaiser, U.; Eigler, S. Brodie’s or Hummers’ Method: Oxidation Conditions Determine the Structure of Graphene Oxide. Chem. Eur. J. 2019, 25, 8955–8959. [Google Scholar] [CrossRef]
- Fu, J.; Wei, C.; Wang, W.; Wei, J.L.; Lv, J. Studies of structure and properties of graphene oxide prepared by ball milling. Mater. Res. Innov. 2015, 19, S277–S280. [Google Scholar] [CrossRef]
- Wei, B.; Cheng, X.; Wang, G.; Li, H.; Song, X.; Dai, L. Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution. Appl. Sci. 2019, 9, 1390. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, C.H.; Yang, Y.H.; Gu, J.; Bao, Q.L.; Lu, J.J.; Su, L.J.; Yang, L.J. Efficiency and mechanism of acridine orange removal by citric-acid-crosslinked beta-cyclodextrin. Mater. Lett. 2022, 312, 131688. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Eris, S.; Bashiri, H. Kinetic study of the adsorption of dyes onto activated carbon. Prog. React. Kinet. Mech. 2016, 41, 109–119. [Google Scholar] [CrossRef]
- Arias Arias, F.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Vacacela Gomez, C.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef]
- Chen, F.; Guo, L.; Zhang, X.; Leong, Z.Y.; Yang, S.; Yang, H.Y. Nitrogen-doped graphene oxide for effectively removing boron ions from seawater. Nanoscale 2017, 9, 326–333. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Russo, P.; D’Urso, L.; Hu, A.; Zhou, N.; Compagnini, G. In liquid laser treated graphene oxide for dye removal. Appl. Surf. Sci. 2015, 348, 85–91. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Huang, Y.; Hayat, T.; Alsaedi, A.; Li, J. Interaction mechanisms of U(VI) and graphene oxide from the perspective of particle size distribution. J. Radioanal. Nucl. Chem. 2016, 311, 209–217. [Google Scholar] [CrossRef]
- King, A.A.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; Sun, Y.Y.; Zhang, S.B. Characteristics of Raman spectra for graphene oxide from ab initio simulations. J. Chem. Phys. 2011, 135, 184503. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Shams, M.; Guiney, L.M.; Huang, L.; Ramesh, M.; Yang, X.; Hersam, M.C.; Chowdhury, I. Influence of functional groups on the degradation of graphene oxide nanomaterials. Environ. Sci. Nano 2019, 6, 2203–2214. [Google Scholar] [CrossRef]
- He, G.; Zhang, J.; Zhang, Y.; Chen, H.; Wang, X. Fast and Efficient Removal of Cationic Dye Using Graphite Oxide, Adsorption, and Kinetics Studies. J. Dispers. Sci. Technol. 2013, 34, 1223–1229. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Chu, L. Chlorine-functionalized reduced graphene oxide for methylene blue removal. RSC Adv. 2015, 5, 52466–52472. [Google Scholar] [CrossRef]
- Obraztsova, E.Y.; Barshutina, M.N.; Bakunin, E.S.; Rukhov, A.V.; Shipovskaya, A.A.; Shuklinov, A.V. Adsorption characteristics of nanographite oxide obtained from thermally expanded graphite. Mendeleev Commun. 2020, 30, 174–176. [Google Scholar] [CrossRef]
- Hernandez, C.N.; Garcia, M.B.G.; Santos, D.H.; Heras, M.A.; Colina, A.; Fanjul-Bolado, P. Aqueous UV-VIS spectroelectrochemical study of the voltammetric reduction of graphene oxide on screen-printed carbon electrodes. Electrochem. Commun. 2016, 64, 65–68. [Google Scholar] [CrossRef]
- Ding, J.N.; Liu, Y.B.; Yuan, N.Y.; Ding, G.Q.; Fan, Y.; Yu, C.T. The influence of temperature, time and concentration on the dispersion of reduced graphene oxide prepared by hydrothermal reduction. Diam. Relat. Mater. 2012, 21, 11–15. [Google Scholar] [CrossRef]
- Chandu, B.; Mosali, V.S.S.; Mullamuri, B.; Bollikolla, H.B. A facile green reduction of graphene oxide using Annona squamosa leaf extract. Carbon Lett. 2017, 21, 74–80. [Google Scholar] [CrossRef]
- Hagos, F.M.; Qian, H.X.; Di, J.; Shan, S.D.; Yang, R.Q.; Li, Y.; Gai, X.K. Rice Husk Hydrochars Prepared with Different Post-treatment Methods for the Adsorption of Dyes and Antibiotics. Bioresources 2022, 17, 725–749. [Google Scholar] [CrossRef]
- Zbair, M.; Anfar, Z.; Ait Ahsaine, H.; El Alem, N.; Ezahri, M. Acridine orange adsorption by zinc oxide/almond shell activated carbon composite: Operational factors, mechanism and performance optimization using central composite design and surface modeling. J. Environ. Manag. 2018, 206, 383–397. [Google Scholar] [CrossRef]
- Chandrika, K.; Chaudhary, A.; Mareedu, T.; Sirisha, U.; Vangalapati, M. Adsorptive removal of acridine orange dye by green tea/copper-activated carbon nanoparticles (Gt/Cu-AC np). Mater. Today Proc. 2021, 44, 2283–2289. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y. Magnetic graphene oxide modified by chloride imidazole ionic liquid for the high-efficiency adsorption of anionic dyes. RSC Adv. 2017, 7, 9079–9089. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Chen, Y.; Li, Y. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresour. Technol. 2020, 315, 123834. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Mass production of graphene oxide from expanded graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef]
- Bu, J.; Yuan, L.; Zhang, N.; Meng, Y.; Peng, X. Novel Adsorbent of N-Phenylthiourea-Functionalized Graphene Oxide and Its Removal of Methyl Orange in Aqueous Solutions. J. Chem. Eng. Data 2020, 66, 199–209. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Chu, K.H. Revisiting the Temkin Isotherm: Dimensional Inconsistency and Approximate Forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Sun, L.; Chu, Y.; Wang, J.; Wei, C.; Liu, Y.; Jiang, Q.; Han, C.; Yan, H.; Song, X. Ultrasonication-Tailored Graphene Oxide of Varying Sizes in Multiple-Equilibrium-Route-Enhanced Adsorption for Aqueous Removal of Acridine Orange. Molecules 2023, 28, 4179. https://doi.org/10.3390/molecules28104179
Han Z, Sun L, Chu Y, Wang J, Wei C, Liu Y, Jiang Q, Han C, Yan H, Song X. Ultrasonication-Tailored Graphene Oxide of Varying Sizes in Multiple-Equilibrium-Route-Enhanced Adsorption for Aqueous Removal of Acridine Orange. Molecules. 2023; 28(10):4179. https://doi.org/10.3390/molecules28104179
Chicago/Turabian StyleHan, Zhaoyang, Ling Sun, Yingying Chu, Jing Wang, Chenyu Wei, Yifang Liu, Qianlei Jiang, Changbao Han, Hui Yan, and Xuemei Song. 2023. "Ultrasonication-Tailored Graphene Oxide of Varying Sizes in Multiple-Equilibrium-Route-Enhanced Adsorption for Aqueous Removal of Acridine Orange" Molecules 28, no. 10: 4179. https://doi.org/10.3390/molecules28104179
APA StyleHan, Z., Sun, L., Chu, Y., Wang, J., Wei, C., Liu, Y., Jiang, Q., Han, C., Yan, H., & Song, X. (2023). Ultrasonication-Tailored Graphene Oxide of Varying Sizes in Multiple-Equilibrium-Route-Enhanced Adsorption for Aqueous Removal of Acridine Orange. Molecules, 28(10), 4179. https://doi.org/10.3390/molecules28104179