The Role of Short-Chain Fatty Acids in Acute Pancreatitis
Abstract
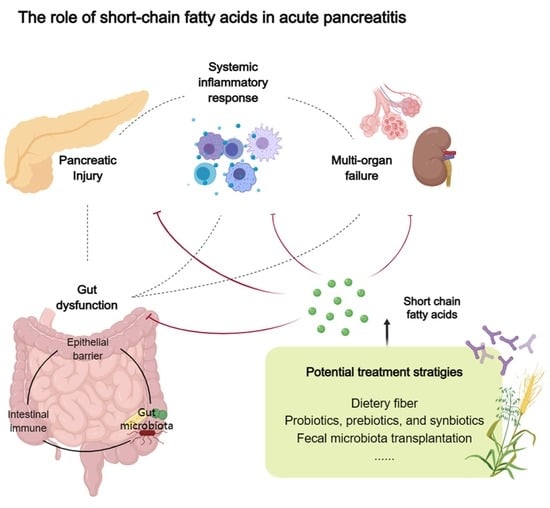
:1. Introduction
2. Alteration of Gut Microecology in AP
| Studies | Subjects | Sample | Phylum | Genus | Species |
|---|---|---|---|---|---|
| Tian 2015 [29] | 76 AP and 32 HC | Fecal | Enterobacteriaceae ↑ Enterococcus ↑ Bifidobacterium ↓ | ||
| Zhang 2018 [17] | 45 AP and 44 HC | Fecal | Bacteroidetes ↑ Proteobacteria ↑ Firmicutes ↓ Actinobacteria ↓ | ||
| Zhu 2019 [18] | 130 AP and 35 HC | Fecal | Proteobacteria ↑ Bacteroidetes ↓ | Escherichia/Shigella ↑ Enterococcus ↑ An unknown genus in family of Enterobacteriaceae ↑ Prevotella_9 ↓ Faecalibacterium ↓ Blautia ↓ Lachnospiraceae ↓ Bifidobacterium ↓ | |
| Yu 2020 [23] | 60 AP and 20 HC | Rectal swab | Finegoldia ↑ Anaerococcus ↑ Enterococcus ↑ Eubacterium hallii ↓ | Blautia ↓ Finegoldia ↑ | |
| van den Berg 2021 [30] | 35 AP and 15 HC | Fecal | Proteobacteria ↑ | Escherichia/Shigella ↑ Streptococcus ↑ Butyrate producers ↓ 1 |
3. Function of SCFAs in AP
3.1. Mitigation of Intestinal Injury
3.2. Reduction of Pancreas Injury
3.3. Prevention and Protection of Other Organ Dysfunctions
4. Treatment Potential of SCFAs in AP
4.1. Dietary Fiber Supplementation
4.2. Probiotics, Prebiotics, and Synbiotics
| Studies | Subjects | Pre/Pro/Synbiotics | Main Effect of Treatment Group |
|---|---|---|---|
| Oláh 2002 [74] | 45 AP | Live L. plantarum 299, together with a substrate of oat fiber | Pancreatic sepsis ↓ Number of surgical interventions ↓ |
| Oláh 2007 [75] | 62 SAP | Four different lactobacilli preparations and prebiotics containing four bioactive fibers (inulin, beta-glucan, resistant starch, and pectin) | Incidence SIRS and MOF ↓ Rate of late (over 48 h) organ failure ↓ |
| Karakan 2007 [73] | 30 SAP | Standard enteral nutrition with soluble and insoluble fibers | Hospital stay ↓ APACHE II, CRP, and CT store normalization duration ↓ Overall complications ↓ |
| Besselink 2008 [76] | 298 predicted SAP | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius, Lactococcus lactis, Bifidobacterium bifidum, and Bifidobacterium lactis | Risk of mortality ↑ |
| Lata 2010 [86] | 22 AP | B. bifidum, B. infantis, L. acidophilus, L. casei, L. salivarius, L. lactis | Endotoxin levels ↓ |
| Sharma 2011 [82] | 50 AP | Lactobacillus acidophilus, Bifidobacterium longus, Bifidobacterium bifidum, and Bifidobacterium infantalis with fructo-oligosaccharide | CRP and immunoglobulins ↓ |
| Plaudis 2012 [87] | 90 SAP | Synbiotic 2000 Forte | Infection rate (pancreatic and peripancreatic necrosis) ↓ Rate of surgical interventions ↓ ICU and hospital stay ↓ Mortality rate ↓ |
| Cui 2013 [81] | 70 SAP | Bifidobacterium | Pro-inflammatory cytokines ↓ Earlier restoration of gastrointestinal function Complications ↓ Hospital day ↓ |
| Wang 2013 [88] | 183 SAP | Live Bacillus subtilis and Enterococcus faecium | Percentage of pancreatic sepsis and MODS ↓ Mortality rate ↓ Pro-inflammatory cytokines and APACHE II scores ↓ Plasma concentration of IL-10 ↑ |
| Zhu 2014 [89] | 39 SAP | C. Butyricum | Rate of intestinal ischemia and necrosis ↑ |
| Li 2014 [90] | 80 SAP | Bifidobacterium | Pro-inflammatory cytokines levels ↓ CRP and LDH levels ↓ Mortality and incidence of complications ↓ Duration of hospitalization ↓ |
| Wu 2017 [91] | 120 SAP | Live B. bifidus, B. acidophilus, E. faecalis, and B. cereus | Incidence of infection MODS ↓ Duration of abdomen pain and hospitalization ↓ |
| Fang 2018 [92] | 68 SAP | Live Bifidobacterium, Lactobacillus, and Enterococcus | Relieved clinical symptoms Hospitalization time ↓ Serum inflammatory cytokine levels ↓ |
| Wang 2023 [84] | 73 MSAP | Lactulose | Serum levels of cytokines ↓ Gut permeability index ↓ Bifidobacterium ↑ Level of SCFAs ↑ |
| Rohith 2023 [85] | 86 MSAP or SAP | Synbiotics containing Streptococcus faecalis T-110, Clostridium butyricum TO-A, Bacillus mesentricus TO-A, and Lactobacillus sporogenes | Total leucocyte and neutrophil counts ↓ Length of hospitalization ↓ |
4.3. Direct Supplementation of SCFAs
4.4. Fecal Microbiota Transplantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, P.A.; Freeman, M.L. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006, 101, 2379–2400. [Google Scholar] [CrossRef]
- Leaphart, C.L.; Tepas, J.J., III. The gut is a motor of organ system dysfunction. Surgery 2007, 141, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br. J. Nutr. 1987, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Nguyen, P.; Ballèvre, O.; Krempf, M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc. Nutr. Soc. 2003, 62, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.J.; Rombeau, J.L. Metabolism and potential clinical applications of short-chain fatty acids. Clin. Nutr. 1993, 12, S97–S105. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Willemsen, L.E.; Koetsier, M.A.; van Deventer, S.J.; van Tol, E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; He, C.; Zhu, Y.; Lu, N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, M.; Albalawi, Z.H.; Tashkandi, M.F.; Al-Ansary, L.A. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst. Rev. 2010, 2010, Cd002837. [Google Scholar] [CrossRef]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, Z.Y.; Zhang, C.H.; Wu, J.; Wang, Y.X.; Zhang, G.X. Intestinal Microbial Community Differs between Acute Pancreatitis Patients and Healthy Volunteers. Biomed. Environ. Sci. 2018, 31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, C.; Li, X.; Cai, Y.; Hu, J.; Liao, Y.; Zhao, J.; Xia, L.; He, W.; Liu, L.; et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J. Gastroenterol. 2019, 54, 347–358. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Jiang, Q.; Han, M.; Zhao, Y.; Long, L.; Fu, C.; Yao, K. Alpha-Ketoglutarate in Low-Protein Diets for Growing Pigs: Effects on Cecal Microbial Communities and Parameters of Microbial Metabolism. Front. Microbiol. 2018, 9, 1057. [Google Scholar] [CrossRef]
- Ma, N.; Wu, Y.; Xie, F.; Du, K.; Wang, Y.; Shi, L.; Ji, L.; Liu, T.; Ma, X. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget 2017, 8, 44625–44638. [Google Scholar] [CrossRef]
- Liu, J.; Yue, S.; Yang, Z.; Feng, W.; Meng, X.; Wang, A.; Peng, C.; Wang, C.; Yan, D. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol. Res. 2018, 134, 40–50. [Google Scholar] [CrossRef]
- Kellingray, L.; Gall, G.L.; Defernez, M.; Beales, I.L.P.; Franslem-Elumogo, N.; Narbad, A. Microbial taxonomic and metabolic alterations during faecal microbiota transplantation to treat Clostridium difficile infection. J. Infect. 2018, 77, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xiong, Y.; Xu, J.; Liang, X.; Fu, Y.; Liu, D.; Yu, X.; Wu, D. Identification of Dysfunctional Gut Microbiota Through Rectal Swab in Patients with Different Severity of Acute Pancreatitis. Dig. Dis. Sci. 2020, 65, 3223–3237. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Bittner, R.; Block, S.; Büchler, M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 1986, 91, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L., III; Allen, K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am. J. Surg. 1991, 161, 19–25. [Google Scholar] [CrossRef]
- van den Berg, F.F.; Hugenholtz, F.; Boermeester, M.A.; Zaborina, O.; Alverdy, J.C. Spatioregional assessment of the gut microbiota in experimental necrotizing pancreatitis. BJS Open 2021, 5, zrab061. [Google Scholar] [CrossRef]
- Tan, C.; Ling, Z.; Huang, Y.; Cao, Y.; Liu, Q.; Cai, T.; Yuan, H.; Liu, C.; Li, Y.; Xu, K. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas 2015, 44, 868–875. [Google Scholar] [CrossRef]
- van den Berg, F.F.; van Dalen, D.; Hyoju, S.K.; van Santvoort, H.C.; Besselink, M.G.; Wiersinga, W.J.; Zaborina, O.; Boermeester, M.A.; Alverdy, J. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut 2021, 70, 915–927. [Google Scholar] [CrossRef]
- Wu, L.M.; Sankaran, S.J.; Plank, L.D.; Windsor, J.A.; Petrov, M.S. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br. J. Surg. 2014, 101, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, H.Y.; Wang, J.Y.; Xiong, H.F.; He, W.H.; Xia, L.; Lu, N.H.; Zhu, Y. Severity of acute gastrointestinal injury grade is a good predictor of mortality in critically ill patients with acute pancreatitis. World J. Gastroenterol. 2020, 26, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Davis, B.; Zhu, W.; Zheng, N.; Meng, D.; Walker, W.A. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G521–G530. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ji, L.; Zhao, Y.; Liu, A.; Wu, D.; Qian, J. Sodium Butyrate Attenuates Taurocholate-Induced Acute Pancreatitis by Maintaining Colonic Barrier and Regulating Gut Microorganisms in Mice. Front. Physiol. 2022, 13, 813735. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Xiao, S.; Jing, S.; Jiakui, S.; Lei, Z.; Ying, L.; Han, L.; Xinwei, M.; Weiqin, L. Butyrate Ameliorates Intestinal Epithelial Barrier Injury Via Enhancing Foxp3+ Regulatory T-Cell Function in Severe Acute Pancreatitis Model. Turk. J. Gastroenterol. 2022, 33, 710–719. [Google Scholar] [CrossRef]
- Harrison, O.J.; Powrie, F.M. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Barnes, M.J.; Powrie, F. Regulatory T cells reinforce intestinal homeostasis. Immunity 2009, 31, 401–411. [Google Scholar] [CrossRef]
- Ke, L.; Ni, H.B.; Sun, J.K.; Tong, Z.H.; Li, W.Q.; Li, N.; Li, J.S. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J. Surg. 2012, 36, 171–178. [Google Scholar] [CrossRef]
- Ke, L.; Tong, Z.H.; Ni, H.B.; Ding, W.W.; Sun, J.K.; Li, W.Q.; Li, N.; Li, J.S. The effect of intra-abdominal hypertension incorporating severe acute pancreatitis in a porcine model. PLoS ONE 2012, 7, e33125. [Google Scholar] [CrossRef]
- Gong, G.; Wang, P.; Ding, W.; Zhao, Y.; Li, J. The role of oxygen-free radical in the apoptosis of enterocytes and bacterial translocation in abdominal compartment syndrome. Free Radic. Res. 2009, 43, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Jia, L.; Yan, Q.Q.; Deng, Q.; Wei, B. Effect of Clostridium butyricum and Butyrate on Intestinal Barrier Functions: Study of a Rat Model of Severe Acute Pancreatitis With Intra-Abdominal Hypertension. Front. Physiol. 2020, 11, 561061. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Jia, L.; Wen, B.; Wu, Y.; Zeng, Y.; Wang, Q. Clostridium butyricum Protects Against Pancreatic and Intestinal Injury After Severe Acute Pancreatitis via Downregulation of MMP9. Front. Pharmacol. 2022, 13, 919010. [Google Scholar] [CrossRef] [PubMed]
- Kocael, A.; Inal, B.B.; Guntas, G.; Kelten, C.; Inal, H.; Topac, H.I.; Kocael, P.; Simsek, O.; Karaca, G.; Salihoglu, Z.; et al. Evaluation of matrix metalloproteinase, myeloperoxidase, and oxidative damage in mesenteric ischemia-reperfusion injury. Hum. Exp. Toxicol. 2016, 35, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Jiang, C.; Pan, K.; Deng, J.; Wan, C. MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immun. 2020, 26, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Paraskeva, E.; Gourgoulianis, K.; Molyvdas, P.A.; Hatzoglou, C. Matrix metalloproteinases 2 and 9 increase permeability of sheep pleura in vitro. BMC Physiol. 2012, 12, 2. [Google Scholar] [CrossRef]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Fang, X.; Wang, F.; Li, H.; Niu, W.; Liang, W.; Wu, C.; Li, J.; Tu, X.; Pan, L.L.; et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br. J. Pharmacol. 2019, 176, 4446–4461. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Yamada, T.; Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Yamakawa, K.; Hamasaki, T.; Nakahori, Y.; Ohnishi, M.; Kuwagata, Y.; et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. JPEN J. Parenter. Enteral Nutr. 2015, 39, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xia, M.; Zhan, Q.; Zhou, Q.; Lu, G.; An, F. Sodium Butyrate Reduces Organ Injuries in Mice with Severe Acute Pancreatitis Through Inhibiting HMGB1 Expression. Dig. Dis. Sci. 2015, 60, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Weng, T.; Shen, K.; Chen, Z.; Yu, Y.; Huang, Q.; Wang, G.; Liu, Z.; Jin, S. The Inflammation Induced by Lipopolysaccharide can be Mitigated by Short-chain Fatty Acid, Butyrate, through Upregulation of IL-10 in Septic Shock. Scand. J. Immunol. 2017, 85, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.L.; Daggett, W.M.; Civette, J.M.; Vasu, M.A.; Lawson, D.W.; Warshaw, A.L.; Nardi, G.L.; Bartlett, M.K. Acute pancreatitis: Analysis of factors influencing survival. Ann. Surg. 1977, 185, 43–51. [Google Scholar] [CrossRef]
- Shields, C.J.; Winter, D.C.; Redmond, H.P. Lung injury in acute pancreatitis: Mechanisms, prevention, and therapy. Curr. Opin. Crit. Care 2002, 8, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L65–L78. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Hellman, J.; Horswill, A.R.; Crosby, H.A.; Francis, K.P.; Prakash, A. Elevated Gut Microbiome-Derived Propionate Levels Are Associated With Reduced Sterile Lung Inflammation and Bacterial Immunity in Mice. Front. Microbiol. 2019, 10, 159. [Google Scholar] [CrossRef]
- Prakash, A.; Sundar, S.V.; Zhu, Y.G.; Tran, A.; Lee, J.W.; Lowell, C.; Hellman, J. Lung Ischemia-Reperfusion is a Sterile Inflammatory Process Influenced by Commensal Microbiota in Mice. Shock 2015, 44, 272–279. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.X.; Hong, M.; Huang, X.Z.; Chen, H.; Xu, J.H.; Wang, C.; Zhang, Y.X.; Zhong, J.X.; Nie, H.; et al. Sodium butyrate alleviates LPS-induced acute lung injury in mice via inhibiting HMGB1 release. Int. Immunopharmacol. 2018, 56, 242–248. [Google Scholar] [CrossRef]
- Liu, J.; Chang, G.; Huang, J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor κB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, C.; Li, N.; Wang, J.; Zhang, Y.; Deng, X. Intraperitoneal Injection of Acetate Protects Mice Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury Through Its Anti-Inflammatory and Anti-Oxidative Ability. Med. Sci. Monit. 2019, 25, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Karoor, V.; Strassheim, D.; Sullivan, T.; Verin, A.; Umapathy, N.S.; Dempsey, E.C.; Frank, D.N.; Stenmark, K.R.; Gerasimovskaya, E. The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 9916. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Han, Z.; Zhou, R.; Su, W.; Gong, L.; Yang, Z.; Song, X.; Zhang, S.; Shu, H.; Wu, D. Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome. Front. Cell. Infect. Microbiol. 2023, 13, 1127369. [Google Scholar] [CrossRef] [PubMed]
- Devani, K.; Charilaou, P.; Radadiya, D.; Brahmbhatt, B.; Young, M.; Reddy, C. Acute pancreatitis: Trends in outcomes and the role of acute kidney injury in mortality- A propensity-matched analysis. Pancreatology 2018, 18, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Nassar, T.I.; Qunibi, W.Y. AKI Associated with Acute Pancreatitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1106–1115. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.J.; Loh, Y.W.; Singer, J.; Zhu, W.; Macia, L.; Mackay, C.R.; Wang, W.; Chadban, S.J.; Wu, H. Fiber Derived Microbial Metabolites Prevent Acute Kidney Injury Through G-Protein Coupled Receptors and HDAC Inhibition. Front. Cell Dev. Biol. 2021, 9, 648639. [Google Scholar] [CrossRef]
- Besselink, M.G.; van Santvoort, H.C.; Boermeester, M.A.; Nieuwenhuijs, V.B.; van Goor, H.; Dejong, C.H.; Schaapherder, A.F.; Gooszen, H.G. Timing and impact of infections in acute pancreatitis. Br. J. Surg. 2009, 96, 267–273. [Google Scholar] [CrossRef]
- Marik, P.E.; Zaloga, G.P. Early enteral nutrition in acutely ill patients: A systematic review. Crit. Care Med. 2001, 29, 2264–2270. [Google Scholar] [CrossRef]
- O’Keefe, S.J.; Ou, J.; Delany, J.P.; Curry, S.; Zoetendal, E.; Gaskins, H.R.; Gunn, S. Effect of fiber supplementation on the microbiota in critically ill patients. World J. Gastrointest. Pathophysiol. 2011, 2, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S.; Whelan, K. Comparison of complications attributable to enteral and parenteral nutrition in predicted severe acute pancreatitis: A systematic review and meta-analysis. Br. J. Nutr. 2010, 103, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Karakan, T.; Ergun, M.; Dogan, I.; Cindoruk, M.; Unal, S. Comparison of early enteral nutrition in severe acute pancreatitis with prebiotic fiber supplementation versus standard enteral solution: A prospective randomized double-blind study. World J. Gastroenterol. 2007, 13, 2733–2737. [Google Scholar] [CrossRef]
- Oláh, A.; Belágyi, T.; Issekutz, A.; Gamal, M.E.; Bengmark, S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br. J. Surg. 2002, 89, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Belágyi, T.; Pótó, L.; Romics, L., Jr.; Bengmark, S. Synbiotic control of inflammation and infection in severe acute pancreatitis: A prospective, randomized, double blind study. Hepatogastroenterology 2007, 54, 590–594. [Google Scholar]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Heyland, D.K.; Wischmeyer, P.E. Comment on: Probiotic prophylaxis in predicted severe acute pancreatitis: A randomized, double-blind, placebo-controlled trial. JPEN J. Parenter. Enteral Nutr. 2009, 33, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, J.R.; McClave, S.A. Controversial results with use of probiotics in critical illness: Contradictory findings from large multicenter trial. Curr. Gastroenterol. Rep. 2009, 11, 259–262. [Google Scholar] [CrossRef]
- Expression of concern—Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 875–876. [CrossRef]
- Reddy, B.S.; MacFie, J. Probiotic prophylaxis in predicted severe acute pancreatitis. Lancet 2008, 372, 113. [Google Scholar] [CrossRef]
- Cui, L.H.; Wang, X.H.; Peng, L.H.; Yu, L.; Yang, Y.S. [The effects of early enteral nutrition with addition of probiotics on the prognosis of patients suffering from severe acute pancreatitis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013, 25, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Srivastava, S.; Singh, N.; Sachdev, V.; Kapur, S.; Saraya, A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: A double-blind randomized controlled trial. J. Clin. Gastroenterol. 2011, 45, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y.; Yang, Q.; Lee, P.; Windsor, J.A.; Wu, D. An Updated Systematic Review With Meta-analysis: Efficacy of Prebiotic, Probiotic, and Synbiotic Treatment of Patients With Severe Acute Pancreatitis. Pancreas 2021, 50, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, M.; Hu, Y.; Lei, Y.; Zhu, Y.; Xiong, H.; He, C. Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction. Biomed. Pharmacother. 2023, 163, 114769. [Google Scholar] [CrossRef] [PubMed]
- Rohith, G.; Sureshkumar, S.; Anandhi, A.; Kate, V.; Rajesh, B.S.; Abdulbasith, K.M.; Nanda, N.; Palanivel, C.; Vijayakumar, C. Effect of Synbiotics in Reducing the Systemic Inflammatory Response and Septic Complications in Moderately Severe and Severe Acute Pancreatitis: A Prospective Parallel-Arm Double-Blind Randomized Trial. Dig. Dis. Sci. 2023, 68, 969–977. [Google Scholar] [CrossRef]
- Lata, J.; Juránková, J.; Stibůrek, O.; Príbramská, V.; Senkyrík, M.; Vanásek, T. [Probiotics in acute pancreatitis—A randomised, placebo-controlled, double-blind study]. Vnitr. Lek. 2010, 56, 111–114. [Google Scholar]
- Plaudis, H.; Pupelis, G.; Zeiza, K.; Boka, V. Early low volume oral synbiotic/prebiotic supplemented enteral stimulation of the gut in patients with severe acute pancreatitis: A prospective feasibility study. Acta Chir. Belg. 2012, 112, 131–138. [Google Scholar] [CrossRef]
- Wang, G.; Wen, J.; Xu, L.; Zhou, S.; Gong, M.; Wen, P.; Xiao, X. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J. Surg. Res. 2013, 183, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M. Effects of probiotics in treatment of severe acute pancreatitis. World Chin. J. Dig. 2014, 22, 5013. [Google Scholar] [CrossRef]
- Jin, L. Effect of early enteral nutrition with Bifico on levels of inflammatory mediators in plasma of patients with severe acute pancreatitis. World Chin. J. Dig. 2014, 22, 5609. [Google Scholar]
- Wu, P.; Yu, Y.; Li, L.; Sun, W. Effect and safety of probiotics combined early enteral nutrition on severe acute pancreatitis patients. Biomed. Res. 2017, 28, 1403–1407. [Google Scholar]
- Fang, J.J.; Qin, H.; Shi, C.L.; Jing, T.; Yan, B.Q.; Gai, L.; Li, X.G.; Unit, I.C. Effect of probiotics plus antibiotics on inflammatory cytokines and quality of life in patients with non-biliary severe acute pancreatitis. World Chin. J. Dig. 2018, 26, 270–275. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, A.H.; Brzezinski, A.; Baker, J.P. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am. J. Gastroenterol. 1994, 89, 179–183. [Google Scholar]
- Scheppach, W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig. Dis. Sci. 1996, 41, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpiani, D.; Di Paolo, M.C.; Paoluzi, P.; Torsoli, A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, A.H.; Hiruki, T.; Brzezinski, A.; Baker, J.P. Treatment of left-sided ulcerative colitis with butyrate enemas: A controlled trial. Aliment. Pharmacol. Ther. 1996, 10, 729–736. [Google Scholar] [CrossRef]
- Patz, J.; Jacobsohn, W.Z.; Gottschalk-Sabag, S.; Zeides, S.; Braverman, D.Z. Treatment of refractory distal ulcerative colitis with short chain fatty acid enemas. Am. J. Gastroenterol. 1996, 91, 731–734. [Google Scholar]
- Talley, N.A.; Chen, F.; King, D.; Jones, M.; Talley, N.J. Short-chain fatty acids in the treatment of radiation proctitis: A randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis. Colon Rectum 1997, 40, 1046–1050. [Google Scholar] [CrossRef]
- Pinto, A.; Fidalgo, P.; Cravo, M.; Midões, J.; Chaves, P.; Rosa, J.; dos Anjos Brito, M.; Leitão, C.N. Short chain fatty acids are effective in short-term treatment of chronic radiation proctitis: Randomized, double-blind, controlled trial. Dis. Colon Rectum 1999, 42, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Fracasso, P.L.; Casale, V.; Villotti, G.; Marcheggiano, A.; Stigliano, V.; Pinnaro, P.; Bagnardi, V.; Caprilli, R. Topical butyrate for acute radiation proctitis: Randomised, crossover trial. Lancet 2000, 356, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Magli, A.; Rancati, T.; Fiorino, C.; Valvo, F.; Fellin, G.; Ricardi, U.; Munoz, F.; Cosentino, D.; Cazzaniga, L.F.; et al. Daily sodium butyrate enema for the prevention of radiation proctitis in prostate cancer patients undergoing radical radiation therapy: Results of a multicenter randomized placebo-controlled dose-finding phase 2 study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 518–524. [Google Scholar] [CrossRef]
- Vanhoutvin, S.A.; Troost, F.J.; Kilkens, T.O.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.; Venema, K.; Brummer, R.J. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 952-e76. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Nicholson, M.R.; Hourigan, S.K.; Conrad, M.; Goyal, A.; Jensen, K.; Kelsen, J.; Kennedy, M.; Weatherly, M.; Kahn, S.A. Current Challenges in Fecal Microbiota Transplantation for Clostridioides difficile Infection in Children. Am. J. Gastroenterol. 2021, 116, 1954–1956. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, H.Y.; He, C.; Lv, N.H.; Zhu, L. Fecal microbiota transplantation as an effective initial therapy for pancreatitis complicated with severe Clostridium difficile infection: A case report. World J. Clin. Cases 2019, 7, 2597–2604. [Google Scholar] [CrossRef]
- Liu, L.W.; Xie, Y.; Li, G.Q.; Zhang, T.; Sui, Y.H.; Zhao, Z.J.; Zhang, Y.Y.; Yang, W.B.; Geng, X.L.; Xue, D.B.; et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br. J. Pharmacol. 2023, 180, 647–666. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Li, J.; Wu, D. The Role of Short-Chain Fatty Acids in Acute Pancreatitis. Molecules 2023, 28, 4985. https://doi.org/10.3390/molecules28134985
Yan X, Li J, Wu D. The Role of Short-Chain Fatty Acids in Acute Pancreatitis. Molecules. 2023; 28(13):4985. https://doi.org/10.3390/molecules28134985
Chicago/Turabian StyleYan, Xiaxiao, Jianing Li, and Dong Wu. 2023. "The Role of Short-Chain Fatty Acids in Acute Pancreatitis" Molecules 28, no. 13: 4985. https://doi.org/10.3390/molecules28134985
APA StyleYan, X., Li, J., & Wu, D. (2023). The Role of Short-Chain Fatty Acids in Acute Pancreatitis. Molecules, 28(13), 4985. https://doi.org/10.3390/molecules28134985