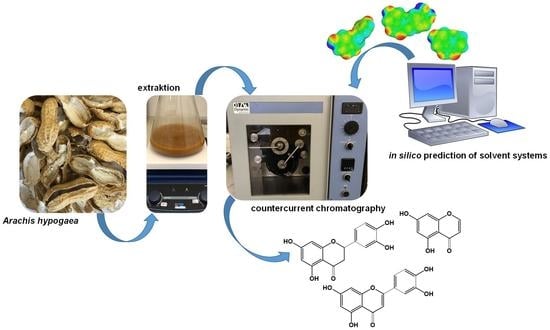
A Comparison between High-Performance Countercurrent Chromatography and Fast-Centrifugal Partition Chromatography for a One-Step Isolation of Flavonoids from Peanut Hulls Supported by a Conductor like Screening Model for Real Solvents
Abstract
:1. Introduction
1.1. Ingredients and Antioxidative Effects of Peanut Hulls
1.2. Liquid-Liquid Chromatography Separation Techniques
1.3. COSMO-RS Supported Solvent System Selection
2. Results and Discussion
2.1. Extraction and Pre-Analysis of Polyphenols in Extract of Roasted Peanut Hulls
2.2. Evaluation and Prediction of Compound Specific Partition Ratio KD-Values by HPLC-UV Analysis and COSMO-RS Calculation of Roasted Peanut Hull Extract
2.3. Isolation of Luteolin, Eriodictyol and 5,7-Dihydroxychromone from Roasted Peanut Hulls by Semi-Preparative HPCCC/FCPC Fractionation and HPLC-Purification
2.4. Comparison of COSMO-RS and HPLC-UV Based Shake Flask Prediction vs. Experimental HPCCC and FCPC KD-Values
2.5. Comparing Chromatographic Performance of HPCCC and FCPC
3. Material and Methods
3.1. Chemicals
3.2. Peanut Hull Preparation and Extraction
3.3. Computational Calculation of Phase Equilibrium and KD-Values Using COSMO-RS Software
3.4. HPCCC Separation Procedure
3.4.1. Selection of the Solvent System by Shake-Flask Experiments
3.4.2. HPCCC Apparatus and Separations
3.5. FCPC Apparatus and Separation Procedure
3.6. Analysis of HPCCC Fractions by TLC
3.7. HPLC-UV Analyses
3.8. Preparative HPLC Separations
3.9. HPLC-UV-MS Analyses
3.10. Spectroscopic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- USDA Foreign Agricultural Service. Erntemenge von Erdnüssen Weltweit in den Jahren 2013/14 bis 2022/23 (in Millionen Tonnen). Available online: https://de.statista.com/statistik/daten/studie/516661/umfrage/erntemenge-von-erdnuessen-weltweit/ (accessed on 28 April 2023).
- Zhao, X.; Chen, J.; Du, F. Potential Use of Peanut By-Products in Food Processing: A Review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhu, Q.; Wang, D.; Wang, W.; Sun, X.; Liu, X.; Du, F. Screening Natural Antioxidants in Peanut Shell Using DPPH–HPLC–DAD–TOF/MS Methods. Food Chem. 2012, 135, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Igarashi, K.; Li, Y.L. Anti-Diabetic Effects of Luteolin and Luteolin-7-O-Glucoside on KK-Ay Mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.-J.; Shi, J.-Z.; He, Y.-Y.; Hao, J.-J.; Zhang, H.-Y.; Luo, D.-M.; Song, J.-K.; Yan, Y.; Xie, X.-M.; Du, G.-H.; et al. Luteolin Alleviates Cognitive Impairment in Alzheimer’s Disease Mouse Model via Inhibiting Endoplasmic Reticulum Stress-Dependent Neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Lee, J.J.; Kim, Y.; Kim, I.S.; Han, J.H.; Lee, S.G.; Ahn, M.J.; Jung, S.H.; Myung, C.S. Effect of Eriodictyol on Glucose Uptake and Insulin Resistance in Vitro. J. Agric. Food Chem. 2012, 60, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-ΚB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef]
- Gao, A.X.; Xiao, J.; Xia, T.C.-X.; Dong, T.T.-X.; Tsim, K.W.-K. The Extract of Peanut Shell Enhances Neurite Outgrowth of Neuronal Cells: Recycling of Agricultural Waste for Development of Nutraceutical Products. J. Funct. Foods 2022, 91, 105023. [Google Scholar] [CrossRef]
- Daigle, D.J.; Conkerton, E.J.; Sanders, T.H.; Mixon, A.C. Peanut Hull Flavonoids: Their Relationship with Peanut Maturity. J. Agric. Food Chem. 1988, 36, 1179–1181. [Google Scholar] [CrossRef]
- Wee, J.-H.; Moon, J.-H.; Eun, J.-B.; Chung, J.-H.; Kim, Y.-G.; Park, K.-H. Isolation and Identification of Antioxidants from Peanut Shells and the Relationship between Structure and Antioxidant Activity. Food Sci. Biotechnol. 2007, 16, 116–122. [Google Scholar]
- Niu, D.; Liu, C.; Liu, X.; Li, M. Preparative Separation of Flavones from Peanut Hull by High-Speed Counter-Current Chromatography. Nat. Prod. Res. Dev. 2011, 23, 110–113. [Google Scholar]
- Delannay, E.; Toribio, A.; Boudesocque, L.; Nuzillard, J.-M.; Zèches-Hanrot, M.; Dardennes, E.; Le Dour, G.; Sapi, J.; Renault, J.-H. Multiple Dual-Mode Centrifugal Partition Chromatography, a Semi-Continuous Development Mode for Routine Laboratory-Scale Purifications. J. Chromatogr. A 2006, 1127, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.A. Recent Progress on the Industrial Scale-up of Counter-Current Chromatography. J. Chromatogr. A 2007, 1151, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden Rules and Pitfalls in Selecting Optimum Conditions for High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Jerz, G.; Winterhalter, P. Preparation of Three Flavonoids from the Bark of Salix Alba by High-Speed Countercurrent Chromatographic Separation. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 3257–3264. [Google Scholar] [CrossRef]
- Dai, W.; Ramos-Jerz, M.; Xie, D.; Peng, J.; Winterhalter, P.; Jerz, G.; Lin, Z. Isolation of N-Ethyl-2-Pyrrolidinone-Substituted Flavanols from White Tea Using Centrifugal Countercurrent Chromatography off-Line ESI-MS Profiling and Semi-Preparative Liquid Chromatography. Molecules 2021, 26, 7284. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.A.; Medina-Bolivar, F.; Martin, E.M.; Engelberth, A.S.; Villagarcia, H.; Clausen, E.C.; Carrier, D.J. Purification of Resveratrol, Arachidin-1, and Arachidin-3 from Hairy Root Cultures of Peanut (Arachis Hypogaea) and Determination of Their Antioxidant Activity and Cytotoxicity. Biotechnol. Prog. 2010, 26, 1344–1351. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wray, V.; Winterhalter, P. Isolation of Dimeric, Trimeric, Tetrameric and Pentameric Procyanidins from Unroasted Cocoa Beans (Theobroma cacao L.) Using Countercurrent Chromatography. Food Chem. 2015, 179, 278–289. [Google Scholar] [CrossRef]
- Foucault, A.P. Centrifugal Partition Chromatography; Marcel Dekker, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Das Neves Costa, F.; Vieira, M.N.; Garrard, I.; Hewitson, P.; Jerz, G.; Leitão, G.G.; Ignatova, S. Schinus Terebinthifolius Countercurrent Chromatography (Part II): Intra-Apparatus Scale-up and Inter-Apparatus Method Transfer. J. Chromatogr. A 2016, 1466, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the Flowers of Impatiens Glandulifera Royle Isolated by High Performance Countercurrent Chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef]
- DeAmicis, C.; Edwards, N.A.; Giles, M.B.; Harris, G.H.; Hewitson, P.; Janaway, L.; Ignatova, S. Comparison of Preparative Reversed Phase Liquid Chromatography and Countercurrent Chromatography for the Kilogram Scale Purification of Crude Spinetoram Insecticide. J. Chromatogr. A 2011, 1218, 6122–6127. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- BIOVIA COSMOtherm, Release 2021. Dassault Systèmes. Available online: http://www.3ds.com (accessed on 10 June 2023).
- Eckert, F.; Klamt, A. Fast Solvent Screening via Quantum Chemistry: COSMO-RS Approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Berthod, A.; Hassoun, M.; Ruiz-Angel, M.J. Alkane Effect in the Arizona Liquid Systems Used in Countercurrent Chromatography. Anal. Bioanal. Chem. 2005, 383, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Hopmann, E.; Arlt, W.; Minceva, M. Solvent System Selection in Counter-Current Chromatography Using Conductor-like Screening Model for Real Solvents. J. Chromatogr. A 2011, 1218, 242–250. [Google Scholar] [CrossRef]
- Hopmann, E.; Frey, A.; Minceva, M. A Priori Selection of the Mobile and Stationary Phase in Centrifugal Partition Chromatography and Counter-Current Chromatography. J. Chromatogr. A 2012, 1238, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Hopmann, E.; Minceva, M. Selection of Biphasic Liquid Systems in Liquid-Liquid Chromatography Using Predictive Thermodynamic Models. Chem. Eng. Technol. 2014, 37, 1663–1674. [Google Scholar] [CrossRef]
- Brace, E.C.; Engelberth, A.S. Enhancing Silymarin Fractionation Using the Conductor-like Screening Model for Real Solvents. J. Chromatogr. A 2017, 1487, 187–193. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Fan, C.; Cao, X. One-Step Preparative Separation of Flavone and Isoflavone Glycosides from Sophora Japonica Fruit by High-Speed Counter-Current Chromatography Based on COSMO-RS Model. Int. J. Nutr. Sci. 2021, 6, 148–154. [Google Scholar] [CrossRef]
- Luca, S.V.; Roehrer, S.; Kleigrewe, K.; Minceva, M. Approach for Simultaneous Cannabidiol Isolation and Pesticide Removal from Hemp Extracts with Liquid-Liquid Chromatography. Ind. Crops Prod. 2020, 155, 112726. [Google Scholar] [CrossRef]
- Brent Friesen, J.; Pauli, G.F. G.U.E.S.S.—A Generally Useful Estimate of Solvent Systems for CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2777–2806. [Google Scholar] [CrossRef]
- Oka, F.; Oka, H.; Ito, Y. Systematic Search for Suitable Two-Phase Solvent Systems for High-Speed Counter-Current Chromatography. J. Chromatogr. A 1991, 538, 99–108. [Google Scholar] [CrossRef]
- Berthod, A.; Friesen, J.B.; Inui, T.; Pauli, G.F. Elution−Extrusion Countercurrent Chromatography: Theory and Concepts in Metabolic Analysis. Anal. Chem. 2007, 79, 3371–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Van Lehn, R.C. Solvent Selection for the Separation of Lignin-Derived Monomers Using the Conductor-like Screening Model for Real Solvents. Ind. Eng. Chem. Res. 2020, 59, 7755–7764. [Google Scholar] [CrossRef]
- Stahl, E.; Kaltenbach, U. Dünnschicht-Chromatographie: VI. Mitteilung. Spurenanalyse von Zuckergemischen Auf Kieselgur G-Schichten. J. Chromatogr. A 1961, 5, 351–355. [Google Scholar] [CrossRef]
- Ley, J.P.; Krammer, G.; Reinders, G.; Gatfield, I.L.; Bertram, H.-J. Evaluation of Bitter Masking Flavanones from Herba Santa (Eriodictyon californicum (H. & A.) Torr., Hydrophyllaceae). J. Agric. Food Chem. 2005, 53, 6061–6066. [Google Scholar] [CrossRef] [PubMed]





| System | [15] * | [26] * | [33] * | [34] * | n-Hexane | Ethyl Acetate | Methanol | Water |
|---|---|---|---|---|---|---|---|---|
| 1 | Q | −3 | 1.5 | 1.0 | 1.5 | 1.0 | ||
| 2 | N | 0 | 6 | 1.0 | 1.0 | 1.0 | 1.0 | |
| 3 | 1 | 4.0 | 6.0 | 5.0 | 5.0 | |||
| 4 | 1 | 1.0 | 1.0 | 1.0 | 1.5 | |||
| 5 | L | 3 | 1.0 | 1.5 | 1.0 | 1.5 | ||
| 6 | 4 | 3.0 | 7.0 | 4.0 | 6.0 | |||
| 7 | K | 1.0 | 2.0 | 1.0 | 2.0 | |||
| 8 | H | 1.0 | 3.0 | 1.0 | 3.0 | |||
| 9 | G | 6 | 1.0 | 4.0 | 1.0 | 4.0 | ||
| 10 | C | 7 | 1.0 | 9.0 | 1.0 | 9.0 |
| Solvent Systems Calculated by COSMO-RS | Klut | Keri | Kdih | αlut/eri | αeri/dih | αdih/eri |
|---|---|---|---|---|---|---|
| 1 | 0.01 | 0.02 | 0.13 | 2.00 | 6.50 | - |
| 2 | 0.10 | 0.14 | 0.38 | 1.40 | 2.71 | - |
| 3 | 0.20 | 0.27 | 0.53 | 1.35 | 1.96 | - |
| 4 | 0.30 | 0.47 | 0.75 | 1.57 | 1.60 | - |
| 5 | 0.75 | 1.08 | 1.18 | 1.44 | 1.09 | - |
| 6 | 1.44 | 1.96 | 1.61 | 1.36 | - | 1.22 |
| 7 | 3.30 | 4.93 | 2.78 | 1.49 | - | 1.77 |
| 8 | 24 | 38 | 9 | 1.58 | - | 4.22 |
| 9 | 100 | 160 | 21 | 1.60 | - | 7.62 |
| 10 | 1610 | 2597 | 115 | 1.61 | 22.58 | |
| shake flask exp. system 4 | 0.58 | 0.86 | 1.56 | 1.48 | 1.81 | - |
| Klut | Keri | Kdih | αlut/eri | αeri/dih | |
|---|---|---|---|---|---|
| FCPC | 0.74 | 0.96 | 1.49 | 1.30 | 1.55 |
| HPCCC | 0.54 | 0.68 | 1.14 | 1.26 | 1.68 |
| system 4—COSMO-RS | 0.30 | 0.47 | 0.75 | 1.57 | 1.60 |
| system 4—shake flask exp. | 0.58 | 0.86 | 1.56 | 1.48 | 1.81 |
| HPCCC | Corrected SF | 89% | FCPC | Corrected SF | 96% | ||||
|---|---|---|---|---|---|---|---|---|---|
| Target Compounds | Solv. System 4 KD-Calculation (COSMO-RS) | Exp. Peak Range Fractions Retention Vol. [mL] Peak Width [mL] | KD Range ΔKD Width W |
HPCCC Mean Value KD | ΔKD = cal. KD − exp. KD HPCCC | Exp. Peak Range Fractions Retention Vol. [mL] Peak Width [mL] | KD Range ΔKD Width W |
FCPC Mean Value KD | ΔKD = cal. KD − exp. KD FCPC |
| luteolin (1) | 0.30 | F16–F19 68–80 12 | 0.49–0.59 0.10 | 0.54 | −0.24 | F23–27 138–162 24 | 0.68–0.80 0.12 | 0.74 | −0.44 |
| eriodictyol (2) | 0.47 | F20–F23 84–96 12 | 0.63–0.74 0.11 | 0.68 | −0.21 | F28–F36 168–216 48 | 0.83–1.08 0.25 | 0.96 | −0.49 |
| 5,7-dihydroxy-chromone (3) | 0.75 | F32–F36 132–148 16 | 1.06–1.21 0.15 | 1.14 | −0.39 | F37–61 222–366 144 | 1.11–1.86 0.75 | 1.49 | −0.74 |
| Fractionated Pairs | HPCCC α-Value | HPCCC Resolution Factor Rs | FCPC α-Value | FCPC Resolution Factor Rs |
|---|---|---|---|---|
| A-1 | 1.32 | 1.40 | 1.35 | 1.20 |
| 1-2 | 1.26 | 1.33 | 1.30 | 1.17 |
| 2-B | 1.26 | 1.25 | - | - |
| 2-3 | 1.68 | 3.57 | 1.55 | 1.06 |
| B-3 | 1.33 | 1.67 | - | - |
| 3-C | 1.16 | 1.25 | 1.56 | 1.04 |
| System | Klut | Keri | Kdih | αlut/eri | αeri/dih | αdih/eri |
|---|---|---|---|---|---|---|
| 1 | 0.01 | 0.02 | 0.13 | 2.00 | 6.50 | - |
| 2 | 0.10 | 0.14 | 0.38 | 1.40 | 2.71 | - |
| 3 | 0.20 | 0.27 | 0.53 | 1.35 | 1.96 | - |
| 4 | 0.30 | 0.47 | 0.75 | 1.57 | 1.60 | - |
| 5 | 0.75 | 1.08 | 1.18 | 1.44 | 1.09 | - |
| 6 | 1.44 | 1.96 | 1.61 | 1.36 | - | 1.22 |
| 7 | 3.30 | 4.93 | 2.78 | 1.49 | - | 1.77 |
| 8 | 24 | 38 | 9 | 1.58 | - | 4.22 |
| 9 | 100 | 160 | 21 | 1.60 | - | 7.62 |
| 10 | 1610 | 2597 | 115 | 1.61 | - | 22.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiene, M.; Blum, S.; Jerz, G.; Winterhalter, P. A Comparison between High-Performance Countercurrent Chromatography and Fast-Centrifugal Partition Chromatography for a One-Step Isolation of Flavonoids from Peanut Hulls Supported by a Conductor like Screening Model for Real Solvents. Molecules 2023, 28, 5111. https://doi.org/10.3390/molecules28135111
Kiene M, Blum S, Jerz G, Winterhalter P. A Comparison between High-Performance Countercurrent Chromatography and Fast-Centrifugal Partition Chromatography for a One-Step Isolation of Flavonoids from Peanut Hulls Supported by a Conductor like Screening Model for Real Solvents. Molecules. 2023; 28(13):5111. https://doi.org/10.3390/molecules28135111
Chicago/Turabian StyleKiene, Mats, Svenja Blum, Gerold Jerz, and Peter Winterhalter. 2023. "A Comparison between High-Performance Countercurrent Chromatography and Fast-Centrifugal Partition Chromatography for a One-Step Isolation of Flavonoids from Peanut Hulls Supported by a Conductor like Screening Model for Real Solvents" Molecules 28, no. 13: 5111. https://doi.org/10.3390/molecules28135111
APA StyleKiene, M., Blum, S., Jerz, G., & Winterhalter, P. (2023). A Comparison between High-Performance Countercurrent Chromatography and Fast-Centrifugal Partition Chromatography for a One-Step Isolation of Flavonoids from Peanut Hulls Supported by a Conductor like Screening Model for Real Solvents. Molecules, 28(13), 5111. https://doi.org/10.3390/molecules28135111