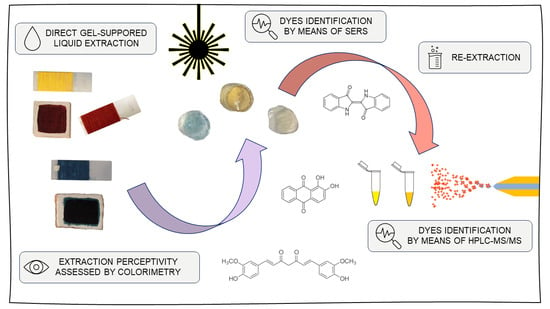
New Advances in Dye Analyses: In Situ Gel-Supported Liquid Extraction from Paint Layers and Textiles for SERS and HPLC-MS/MS Identification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction
2.1.1. Madder and Turmeric
2.1.2. Indigo
2.2. SERS
2.2.1. Madder and Turmeric
2.2.2. Indigo
2.3. HPLC-MS/MS
2.3.1. Madder and Turmeric
2.3.2. Indigo
2.4. Colorimetry
2.4.1. Madder and Turmeric
2.4.2. Indigo
3. Materials and Methods
3.1. Materials
3.2. Lake Pigments Preparation
3.3. Paint Mock-Ups Preparation
3.4. Wool Dyeing
3.5. Agar Gel Preparation
3.6. Ag Colloids Pastes Preparation
3.7. Gel-Supported Liquid Extraction
3.8. SERS Analysis
3.9. dLLME
3.10. HPLC-MS/MS Analyses
3.11. Colorimetric Measurements
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gueli, A.M.; Gallo, S.; Pasquale, S. Optical and colorimetric characterization on binary mixtures prepared with coloured and white historical pigments. Dye. Pigment. 2018, 157, 342–350. [Google Scholar] [CrossRef]
- Peruzzi, G.; Cucci, C.; Picollo, M.; Quercioli, F.; Stefani, L. Non-invasive identification of dyed textiles by using VIS-NIR FORS and hyperspectral imaging techniques. Cult. E Sci. Del Color. Color Cult. Sci. 2021, 13, 61–69. [Google Scholar]
- Degano, I.; Ribechini, E.; Modugno, F.; Colombini, M.P. Analytical Methods for the Characterization of Organic Dyes in Artworks and in Historical Textiles. Appl. Spectrosc. Rev. 2009, 44, 363–410. [Google Scholar] [CrossRef]
- Melo, M.J.; Otero, V.; Vitorino, T.; Araújo, R.; Muralha, S.; Lemos, A.; Picollo, M. A Spectroscopic Study of Brazilwood Paints in Medieval Books of Hours. Appl. Spectrosc. 2014, 68, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Clementi, C.; Basconi, G.; Pellegrino, R.; Romani, A. Carthamus tinctorius L.: A Photophysical Study of the Main Coloured Species for Artwork Diagnostic Purposes. Dye. Pigment. 2014, 103, 127–137. [Google Scholar] [CrossRef]
- Leona, M. Microanalysis of Organic Pigments and Glazes in Polychrome Works of Art by Surface Enhanced Resonance Raman Scattering. Proc. Natl. Acad. Sci. USA 2009, 106, 14757–14762. [Google Scholar] [CrossRef]
- Melo, M.J.; Nabais, P.; Guimarães, M.; Araújo, R.; Castro, R.; Conceição Oliveira, M.; Whitworth, I. Organic Dyes in Illuminated Manuscripts: A Unique Cultural and Historic Record. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160050. [Google Scholar] [CrossRef] [Green Version]
- Tamburini, D.; Dyer, J.; Davit, P.; Aceto, M.; Turina, V.; Borla, M.; Vandenbeusch, M.; Gulmini, M. Compositional and Micro-Morphological Characterisation of Red Colourants in Archaeological Textiles from Pharaonic Egypt. Molecules 2019, 24, 3761. [Google Scholar] [CrossRef] [Green Version]
- Ciccola, A.; Serafini, I.; D’Agostino, G.; Giambra, B.; Bosi, A.; Ripanti, F.; Nucara, A.; Postorino, P.; Curini, R.; Bruno, M. Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach. Heritage 2021, 4, 1807–1820. [Google Scholar] [CrossRef]
- Colantonio, C.; Lanteri, L.; Ciccola, A.; Serafini, I.; Postorino, P.; Censori, E.; Rotari, D.; Pelosi, C. Imaging Diagnostics Coupled with Non-Invasive and Micro-Invasive Analyses for the Restoration of Ethno-graphic Artifacts from French Polynesia. Heritage 2022, 5, 215–232. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Doherty, B.; Brunetti, B.G.; Sgamellotti, A.; Miliani, C. A detachable SERS active cellulose film: A minimally invasive approach to the study of painting lakes. J. Raman Spectrosc. 2011, 42, 1932–1938. [Google Scholar] [CrossRef]
- Leona, M.; Decuzzi, P.; Kubic, T.A.; Gates, G.; Lombardi, J.R. Nondestructive identification of natural and synthetic organic colorants in works of art by surface enhanced Raman scattering. Anal. Chem. 2011, 83, 3990–3993. [Google Scholar] [CrossRef] [PubMed]
- Lofrumento, C.; Ricci, M.; Platania, E.; Becucci, M.; Castellucci, E. SERS detection of red organic dyes in Ag-agar gel. J. Raman Spectrosc. 2013, 44, 47–54. [Google Scholar] [CrossRef]
- Platania, E.; Lombardi, J.R.; Leona, M.; Shibayama, N.; Lofrumento, C.; Ricci, M.; Becucci, M.; Castellucci, E. Suitability of Ag-agar gel for the micro-extraction of organic dyes on different substrates: The case study of wool, silk, printed cotton and a panel painting mock-up. J. Raman Spectrosc. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Platania, E.; Lofrumento, C.; Lottini, E.; Azzaro, E.; Ricci, M.; Becucci, M. Tailored micro-extraction method for Raman/SERS detection of indigoids in ancient textiles. Anal. Bioanal. Chem. 2015, 407, 6505–6514. [Google Scholar] [CrossRef]
- Becucci, M.; Ricci, M.; Lofrumento, C.; Castellucci, E. Identification of organic dyes by surface-enhanced Raman scattering in nano-composite agar-gel matrices: Evaluation of the enhancement factor. Opt. Quantum Electron. 2016, 48, 449. [Google Scholar] [CrossRef]
- Ricci, M.; Lofrumento, C.; Castellucci, E.; Becucci, M. Microanalysis of Organic Pigments in Ancient Textiles by Surface-Enhanced Raman Scattering on Agar Gel Matrices. J. Spectrosc. 2016, 2016, 1380105. [Google Scholar] [CrossRef] [Green Version]
- Germinario, G.; Ciccola, A.; Serafini, I.; Ruggiero, L.; Sbroscia, M.; Vincenti, F.; Fasolato, C.; Curini, R.; Ioele, M.; Postorino, P.; et al. Gel substrates and ammonia-EDTA extraction solution: A new nondestructive combined approach for the identification of anthraquinone dyes from wool textiles. Microchem. J. 2020, 155, 104780. [Google Scholar] [CrossRef]
- Serafini, I.; Lombardi, L.; Vannutelli, L.; Montesano, C.; Sciubba, F.; Guiso, M.; Curini, R.; Bianco, A. How the extraction method could be crucial in the characterization of natural dyes from dyed yarns and lake pigments: The case of American and Armenian cochineal dyes, extracted through the new ammonia-EDTA method. Microchem. J. 2017, 134, 237–245. [Google Scholar] [CrossRef]
- Zhang, X.; Laursen, R.A. Development of Mild Extraction Methods for the Analysis of Natural Dyes in Textiles of Historical Interest Using LC-Diode Array Detector-MS. Anal. Chem. 2005, 77, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, I.; Quye, A.; Trojanowicz, M. Liquid chromatography determination of natural dyes in extracts from historical Scottish textiles excavated from peat bogs. J. Chromatogr. A 2006, 1112, 209–217. [Google Scholar] [CrossRef]
- Sanyova, J. Mild extraction of dyes by hydrofluoric acid in routine analysis of historical paint micro-samples. Microchim. Acta 2008, 162, 361–370. [Google Scholar] [CrossRef]
- Valianou, L.; Karapanagiotis, I.; Chryssoulakis, Y. Comparison of extraction methods for the analysis of natural dyes in historical textiles by high-performance liquid chromatography. Anal. Bioanal. Chem. 2009, 395, 2175–2189. [Google Scholar] [CrossRef]
- Manhita, A.; Ferreira, T.; Candeias, A.; Barrocas Dias, C. Extracting natural dyes from wool-an evaluation of extraction methods. Anal. Bioanal. Chem. 2011, 400, 1501–1514. [Google Scholar] [CrossRef]
- Lech, K.; Jarozs, M. Novel methodology for the extraction and identification of natural dyestuffs in historical textiles by HPLC–UV–Vis–ESI MS. Case study: Chasubles from the Wawel Cathedral collection. Anal. Bioanal. Chem. 2011, 399, 3241–3251. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, L.; Serafini, I.; Guiso, M.; Sciubba, F.; Bianco, A. A New Approach to the Mild Extraction of Madder Dyes from Lake and Textile. Microchem. J. 2015, 126, 373–380. [Google Scholar] [CrossRef]
- Blackburn, R.S.; Bechtold, T.; John, P. The development of indigo reduction methods and pre-reduced indigo products. Color. Technol. 2009, 125, 193–207. [Google Scholar] [CrossRef]
- Whitney, A.V.; Van Duyne, R.P.; Casadio, F. An innovative surface-enhanced Raman spectroscopy (SERS) method for the identification of six historical red lakes and dyestuffs. J. Raman Spectrosc. 2006, 37, 993–1002. [Google Scholar] [CrossRef]
- Amato, F.; Micciché, F.; Cannas, M.; Gelardi, F.; Pignataro, B.; Li Vigni, M.; Agnello, S. Ag nanoparticles agargel nanocomposites for SERS detection of cultural heritage interest pigments. Eur. Phys. J. Plus 2018, 133, 74. [Google Scholar] [CrossRef]
- Lofrumento, C.; Platania, E.; Ricci, M.; Mulana, C.; Becucci, M.; Castellucci, E. The SERS spectra of alizarin and its ionized species: The contribution of the molecular resonance to the spectral enhancement. J. Mol. Struct. 2015, 1090, 98–106. [Google Scholar] [CrossRef]
- Retko, K.; Ropreta, P.; Cerc Korošec, R. Surface-enhanced Raman spectroscopy (SERS) analysis of organic colourants utilising a new UV-photoreduced substrate. J. Raman Spectrosc. 2014, 45, 1140–1146. [Google Scholar] [CrossRef]
- Rambaldi, D.C.; Pozzi, F.; Shibayama, N.; Leona, M.; Preusser, F.D. Surface-enhanced Raman spectroscopy of various madder species on wool fibers: The role of pseudopurpurin in the interpretation of the spectra. J. Raman Spectrosc. 2015, 46, 1073–1081. [Google Scholar] [CrossRef]
- Pozzi, F.; Zaleski, S.; Casadio, F.; Van Duyne, R.P. SERS Discrimination of Closely Related Molecules: A Systematic Study of Natural Red Dyes in Binary Mixtures. J. Phys. Chem. C 2016, 120, 21017–21026. [Google Scholar] [CrossRef]
- Bosi, A.; Ciccola, A.; Serafini, I.; Peruzzi, G.; Nigro, V.; Postorino, P.; Curini, R.; Favero, G. Gel microextraction from hydrophilic paint layers: A comparison between Agar-gel and Nanorestore Gel® HWR for spectroscopic identification of madder. Microchem. J. 2023, 187, 108447. [Google Scholar] [CrossRef]
- Bruni, S.; Guglielmi, V.; Pozzi, F. Historical organic dyes: A surface-enhanced Raman scattering (SERS) spectral database on Ag Lee-Meisel colloids aggregated by NaClO4: SERS database on historical organic dyes. J. Raman Spectrosc. 2011, 42, 1267–1281. [Google Scholar] [CrossRef]
- Mollica Nardo, V.; Aliotta, F.; Mastelloni, M.A.; Ponterio, R.C.; Saija, F.; Trusso, S.; Vasi, C.S. A spectroscopic approach to the study of organic pigments in the field of cultural heritage. Atti Della Accad. Peloritana Pericolanti 2017, 95, A5. [Google Scholar]
- Coralez, G.; Celis, F.; Gómez-Jeria, J.S.; Campos, M.; Cárcamo-Vega, J.J. Raman of indigo on a silver surface. Raman and theoretical characterization of indigo deposited on silicon dioxide-coated and uncoated silver nanoparticles. Spectrosc. Lett. 2017, 50, 316–321. [Google Scholar] [CrossRef]
- Prieto, B.; Sanmartın, B.; Silva, B.; Martınez-Verdu, F. Measuring the Color of Granite Rocks: A Proposed Procedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- Daniels, V.; Deviese, T.; Hacke, M.; Higgitt, C. Technological Insights into Madder Pigment Production in Antiquity. Br. Mus. Tech. Res. Bull. 2014, 8, 13–28. [Google Scholar]
- Marconi, S. Preparazione e finitura delle opere pittoriche. Materiale e metodi, preparazioni e imprimiture, leganti, vernici, cornice; Mursia: Milan, Italy, 1993. [Google Scholar]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Siver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Idone, A.; Gulmini, M.; Henry, A.-I.; Casadio, F.; Chang, L.; Appolonia, L.; Van Duyned, R.P.; Shah, N.C. Silver colloidal pastes for dye analysis of reference and historical textile fibers using direct, extractionless, non-hydrolysis surface-enhanced Raman spectroscopy. Analyst 2013, 138, 5895. [Google Scholar] [CrossRef] [PubMed]
- Saviello, D.; Alyami, A.; Trabace, M.; Giorgi, R.; Baglioni, P.; Mirabile, A.; Iacopino, D. Plasmonic colloidal pastes for surface-enhanced Raman spectroscopy (SERS) of historical felt-tip pens. RSC Adv. 2018, 8, 8365–8371. [Google Scholar] [CrossRef]
- Vincenti, F.; Montesano, C.; Cellucci, L.; Gregori, A.; Fanti, F.; Compagnone, D.; Curini, R.; Sergi, M. Combination of pressurized liquid extraction with dispersive liquid liquid microextraction for the determination of sixty drugs of abuse in hair. J. Chromatogr. A. 2019, 1605, 360348. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of dispersive liquid–liquid microextraction method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Lech, K.; Fornal, E. A Mass Spectrometry-Based Approach for Characterization of Red, Blue, and Purple Natural Dyes. Molecules 2020, 25, 3223. [Google Scholar] [CrossRef] [PubMed]
- Rafaelly, L.; Heron, S.; Nowik, W.; Tchapla, A. Optimisation of ESI-MS detection for the HPLC of anthraquinone dyes. Dye. Pigment. 2008, 77, 191–203. [Google Scholar] [CrossRef]
- Jiang, H.; Timmermann, N.B.; Gang, D.R. Use of liquid chromatography–electrospray ionization tandem mass spectrometry to identify diarylheptanoids in turmeric (Curcuma longa L.) rhizome. J. Chromatogr. A 2006, 1111, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Oleari, C. Misurare il colore: Spettrofotometria, fotometria e colorimetria: Fisiologia e percezione; Casa Editrice Libraria Ulrico Hoepli: Milan, Italy, 1998. [Google Scholar]









| Peaks from Madder | Attribution | Peaks from Turmeric | Attribution | Peaks from the Gel Matrix | |||||
|---|---|---|---|---|---|---|---|---|---|
| Agar Gel (3% w/v) | Nanorestore Gel® HWR | Agar Gel (3% w/v) | Nanorestore Gel® HWR | Agar Gel (3% w/v) | Nanorestore Gel® HWR | ||||
| Tempera | Textile | Tempera | Textile | Textile | Textile | 838 cm−1 1000 cm−1 1034 cm−1 1268 cm−1 1332 cm−1 1383 cm−1 1461 cm−1 | 839 cm−1 999 cm−1 1249 cm−1 1321 cm−1 1355 cm−1 1422 cm−1 1448 cm−1 1760 cm−1 | ||
| 1144 cm−1 | 1155 cm−1 | 1156 cm−1 | 1160 cm−1 | ν C-C, δ C-H | 1139 cm−1 | - | skeleton vibr. | ||
| 1270 cm−1 | 1283 cm−1 | 1290 cm−1 | 1292 cm−1 | ν C-C, δ H-C-C | 1166 cm−1 | skeleton vibr. | |||
| 1320 cm−1 | 1321 cm−1 | 1324 cm−1 | 1315 cm−1 | ν C-C, δ H-C-C | 1290 cm−1 | δ C-C-C, C-C-H and C=CH | |||
| 1615 cm−1 | 1614 cm−1 | 1622 cm−1 | 1611 cm−1 | ν C=O | 1600 cm−1 | δ C-OH | |||
| Peaks from Indigo | Attribution | Peaks from the Gel Matrix | |
|---|---|---|---|
| Tempera | Textile | 675 cm−1 1000 cm−1 1440 cm−1 | |
| 544 cm−1 | 543 cm−1 | δ C=C-CO-C and C-N | |
| 597 cm−1 | 594 cm−1 | δ C-C and C-N | |
| 1224 cm−1 | 1223 cm−1 | δ N-H and C-H | |
| 1250 cm−1 | 1248 cm−1 | δ C-H, C=C, and N-H | |
| 1574 cm−1 | 1573 cm−1 | ν C=C and C=O | |
| Sample | Name | Description | Gel | Extraction Time | ΔE00 |
|---|---|---|---|---|---|
 | R1R | Tempera paint, madder lake pigment | Agar gel (3% w/v) | 15 min | 2.97 |
| Nanorestore Gel® HWR | 15 min | 4.96 | |||
 | ROB | Wool dyed with madder | Agar gel (3% w/v) | 3 h | 2.09 |
| Nanorestore Gel® HWR | 3 h | 2.76 | |||
 | CUR | Wool dyed with turmeric | Agar gel (3% w/v) | 3 h | 3.87 |
| Nanorestore Gel® HWR | 3 h | 5.24 | |||
 | I1AR | Tempera paint, indigo over azurite | Agar gel (3% w/v) | 5 min | 4.14 |
 | IND | Wool dyed with indigo | Agar gel (3% w/v) | 5 min | 1.52 |
| Time (min) | Phase A: 0.1% HCOOH in H2O | Phase B: 0.1% HCOOH in ACN |
|---|---|---|
| 0.00 | 95% | 5% |
| 1.00 | 95% | 5% |
| 5.00 | 35% | 65% |
| 5.20 | 0% | 100% |
| 6.50 | 0% | 100% |
| 7.00 | 95% | 5% |
| 8.50 | 95% | 5% |
| Analyte | Parent Ion (m/z) | Fragments (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
| Alizarin | 239 | 210 | −120 | −8.1 | −49 | −15 |
| 167 | −40 | −10 | ||||
| Purpurin | 255 | 227 | −109 | −8.3 | −38 | −21 |
| 171 | −42 | −13 | ||||
| Curcumin | 367 | 134 | −40 | −2.5 | −48 | −13 |
| 158 | −44 | −14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosi, A.; Peruzzi, G.; Ciccola, A.; Serafini, I.; Vincenti, F.; Montesano, C.; Postorino, P.; Sergi, M.; Favero, G.; Curini, R. New Advances in Dye Analyses: In Situ Gel-Supported Liquid Extraction from Paint Layers and Textiles for SERS and HPLC-MS/MS Identification. Molecules 2023, 28, 5290. https://doi.org/10.3390/molecules28145290
Bosi A, Peruzzi G, Ciccola A, Serafini I, Vincenti F, Montesano C, Postorino P, Sergi M, Favero G, Curini R. New Advances in Dye Analyses: In Situ Gel-Supported Liquid Extraction from Paint Layers and Textiles for SERS and HPLC-MS/MS Identification. Molecules. 2023; 28(14):5290. https://doi.org/10.3390/molecules28145290
Chicago/Turabian StyleBosi, Adele, Greta Peruzzi, Alessandro Ciccola, Ilaria Serafini, Flaminia Vincenti, Camilla Montesano, Paolo Postorino, Manuel Sergi, Gabriele Favero, and Roberta Curini. 2023. "New Advances in Dye Analyses: In Situ Gel-Supported Liquid Extraction from Paint Layers and Textiles for SERS and HPLC-MS/MS Identification" Molecules 28, no. 14: 5290. https://doi.org/10.3390/molecules28145290
APA StyleBosi, A., Peruzzi, G., Ciccola, A., Serafini, I., Vincenti, F., Montesano, C., Postorino, P., Sergi, M., Favero, G., & Curini, R. (2023). New Advances in Dye Analyses: In Situ Gel-Supported Liquid Extraction from Paint Layers and Textiles for SERS and HPLC-MS/MS Identification. Molecules, 28(14), 5290. https://doi.org/10.3390/molecules28145290