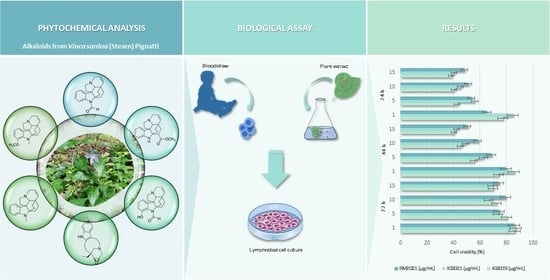
Phytochemical Analysis and In Vitro Antileukemic Activity of Alkaloid-Enriched Extracts from Vinca sardoa (Stearn) Pignatti
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of V. sardoa Aerial Parts
2.2. LC-PDA-ESI-MS and DI-ESI-MS/MS Analysis of the Extracts
2.3. NMR Analysis
2.4. Cytotoxicity Assays
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Extraction Procedure
3.4. LC-PDA-ESI-MS Analysis
3.4.1. Sample Preparation
3.4.2. HPLC-PDA-ESI-MS in SIR Mode Method
3.4.3. DI-ESI-MS/MS Experiments
3.5. NMR Analysis
3.6. Cell Culture
MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Foddai, M.; Maldini, M.; Addis, R.; Petretto, G.L.; Chessa, M.; Pintore, G. Profiling of the bioactive compounds in flowers, leaves and roots of Vinca sardoa. Nat. Prod. Commun. 2017, 12, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2018; Volume 3, pp. 132–133. [Google Scholar]
- Bianco, A.; Guiso, M.; Nicoletti, M.; Foddai, S.; Piccin, A.; Serafini, M.; Ballero, A.; Poli, F. A comparative chemotaxonomic study on Vinca sardoa Stearn and Vinca difformis Pourret. Nat. Prod. Res. 2005, 19, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Danieli, B.; Lesma, G.; Palmisano, G.; Passarella, D.; Vecchietti, V. Indole alkaloids from Vinca sardoa, a new species of Vinca. Heterocycles 1990, 31, 1663–1667. [Google Scholar]
- Nicoletti, M.; Serafini, M.; Federici, E.; Galeffi, C.; Poli, F. Indole alkaloids from aerial parts of Vinca sardoa. Phytochemistry 1998, 47, 149–151. [Google Scholar] [CrossRef]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef] [PubMed]
- Plazas, E.; Faraone, N. Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents. Biomedicines 2023, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.A.; Ramli, A.N.; Yusoff, M.M. Indole alkaloids from plants as potential leads for antidepressant drugs: A mini review. Front. Pharmacol. 2017, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramkissoon, A.; Seepersaud, M.; Maxwell, A.; Jayaraman, J.; Ramsubhag, A. Isolation and Antibacterial Activity of Indole Alkaloids from Pseudomonas aeruginosa UWI-1. Molecules 2020, 25, 3744. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegazy, A.; Mahmoud, S.H.; Elshaier, Y.A.; Shama, N.M.A.; Nasr, N.F.; Ali, M.A.; El-Shazly, A.M.; Mostafa, I.; Mostafa, A. Antiviral activities of plant-derived indole and β-carboline alkaloids against human and avian influenza viruses. Sci. Rep. 2023, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.L.; Huang, W.; Zhu, H.P.; Peng, C.; Zhao, Q.; Han, B. Advances in indole-containing alkaloids as potential anticancer agents by regulating autophagy. Biomed. Pharmacother. 2022, 149, 112827. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, B.; Li, M.; Zhang, J. The current scenario of naturally occurring indole alkaloids with anticancer potential. Fitoterapia 2023, 165, 105430. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Hall, G.W.; McKay, P.; Gallop-Evans, E.; Fielding, P.; Collins, G.P. Management of children and adults with all stages of nodular lymphocyte predominant Hodgkin lymphoma—All StAGEs: A consensus-based position paper from the Hodgkin lymphoma subgroup of the UK National Cancer Research Institute. Br. J. Haematol. 2022, 197, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Medinger, M.; Heim, D.; Lengerke, C.; Halter, J.P.; Passweg, J.R. Akute Lymphoblastische Leukämie–diagnostik und Therapie. Ther. Umsch. 2019, 76, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Herradón, E.; González, C.; González, A.; Uranga, J.A.; López-Miranda, V. Cardiovascular toxicity induced by chronic vincristine treatment. Front. Pharmacol. 2021, 12, 692970. [Google Scholar] [CrossRef]
- Franke, N.E.; Blok, G.J.; Voll, M.L.; Schouten-van Meeteren, A.Y. Transient hepatotoxicity induced by vinblastine in a young girl with chiasmatic low grade glioma. Curr. Drug Saf. 2020, 15, 231–235. [Google Scholar] [CrossRef]
- Maher, T.; Ahmad Raus, R.; Daddiouaissa, D.; Ahmad, F.; Adzhar, N.S.; Latif, E.S.; Abdulhafiz, F.; Mohammed, A. Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules 2021, 26, 2741. [Google Scholar] [CrossRef]
- Sciubba, F.; Tomassini, A.; Giorgi, G.; Brasili, E.; Pasqua, G.; Capuani, G.; Aureli, W.; Miccheli, A. NMR-Based Metabolomic Study of Purple Carrot Optimal Harvest Time for Utilization as a Source of Bioactive Compounds. Appl. Sci. 2020, 10, 8493. [Google Scholar] [CrossRef]
- Frezza, C.; Sciubba, F.; De Vita, D.; Toniolo, C.; Foddai, S.; Tomassini, L.; Petrucci, R.; Bianco, A.; Serafini, M. Non-volatile compounds from Araucaria columnaris (G. Forst.) Hook leaves. Biochem. Syst. Ecol. 2022, 103, 104430. [Google Scholar] [CrossRef]




| Alkaloid | tR (min) | M (Da) | [M + H+]+ (m/z) | Principal Fragments (m/z) | CE (eV) |
|---|---|---|---|---|---|
| 1 | 6.77 ± 0.13 | 306 | 307 | 262, 144 | 30 |
| 2 | 7.04 ± 0.12 | 336 | 337 | 320, 144, 260 | 33 |
| 3 | 7.41 ± 0.17 | 322 | 323 | 278, 160 | 30 |
| 4 | 9.29 ± 0.21 | 296 | 297 | 154, 144, 279 | 28 |
| 5 | 11.74 ± 0.21 | 292 | 293 | 158, 173, 144 | 35 |
| 6 | 12.22 ± 0.15 | 322 | 323 | 188, 203, 174, 144 | 35 |
| RM2019 | IG2019 | IG2021 | |
|---|---|---|---|
| Alkaloids 1, 2, 5 | 177.96 ± 8.89 | 38.7 ± 1.94 | 273.85 ± 13.65 |
| Alkaloid 4 | 167.22 ± 8.36 | 57.66 ± 2.88 | 135.09 ± 6.76 |
| Alkaloids 3, 6 | 177.21 ± 8.86 | 113.89 ± 5.69 | 99.93 ± 4.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vita, D.; Frezza, C.; Sciubba, F.; Toniolo, C.; Badiali, C.; Petrucci, R.; Bortolami, M.; Di Matteo, P.; Rocco, D.; Stringaro, A.; et al. Phytochemical Analysis and In Vitro Antileukemic Activity of Alkaloid-Enriched Extracts from Vinca sardoa (Stearn) Pignatti. Molecules 2023, 28, 5639. https://doi.org/10.3390/molecules28155639
De Vita D, Frezza C, Sciubba F, Toniolo C, Badiali C, Petrucci R, Bortolami M, Di Matteo P, Rocco D, Stringaro A, et al. Phytochemical Analysis and In Vitro Antileukemic Activity of Alkaloid-Enriched Extracts from Vinca sardoa (Stearn) Pignatti. Molecules. 2023; 28(15):5639. https://doi.org/10.3390/molecules28155639
Chicago/Turabian StyleDe Vita, Daniela, Claudio Frezza, Fabio Sciubba, Chiara Toniolo, Camilla Badiali, Rita Petrucci, Martina Bortolami, Paola Di Matteo, Daniele Rocco, Annarita Stringaro, and et al. 2023. "Phytochemical Analysis and In Vitro Antileukemic Activity of Alkaloid-Enriched Extracts from Vinca sardoa (Stearn) Pignatti" Molecules 28, no. 15: 5639. https://doi.org/10.3390/molecules28155639
APA StyleDe Vita, D., Frezza, C., Sciubba, F., Toniolo, C., Badiali, C., Petrucci, R., Bortolami, M., Di Matteo, P., Rocco, D., Stringaro, A., Colone, M., Maxia, A., Petrucci, M. T., Serafini, M., & Foddai, S. (2023). Phytochemical Analysis and In Vitro Antileukemic Activity of Alkaloid-Enriched Extracts from Vinca sardoa (Stearn) Pignatti. Molecules, 28(15), 5639. https://doi.org/10.3390/molecules28155639