New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and Reactivity
Abstract
:1. Introduction
2. Results and Discussion
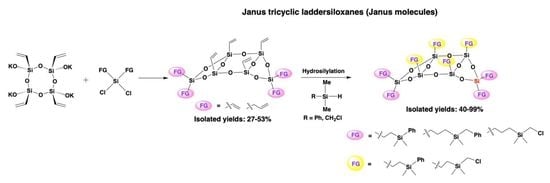
2.1. Preparation of Janus Tricyclic Laddersiloxanes Bearing Eight or Six Alkenyl Groups
2.2. Full Functionalization of Janus Tricyclic Laddersiloxanes Bearing Eight or Six Alkenyl Groups
2.3. Thermal Properties of Laddersiloxanes 6–14
2.4. Trials of Selective Functionalization of Janus Tricyclic Laddersiloxane 7
3. Materials and Methods
3.1. General Considerations
3.2. Synthetic Procedures of Compounds 6–15
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baney, R.H.; Itoh, M.; Sakakibara, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Kickelbick, G. Silsesquioxanes. In Functional Molecular Silicon Compounds I; Structure and Bonding; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 155, pp. 1–28. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.M.; Roll, M.F. Polyhedral Phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2017, 21, 2794–2813. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes—Silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]
- Kim, M.J.; Heo, Y.M.; Cho, J.H. Ladder-type silsesquioxane copolymer gate dielectrics for gating solution-processed IGZO field-effect transistors. Org. Electron. 2017, 43, 41–46. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Takanashi, M.; Imai, T.; Unno, M. Refractive Indices of Silsesquioxanes with Various Structures. Silicon 2015, 7, 127–132. [Google Scholar] [CrossRef]
- Brown, J.F., Jr.; Vogt, L.H., Jr.; Katchman, A.; Eustance, J.W.; Kiser, K.M.; Krantz, K.W. Double Chain Polymers of Phenylsilsesquioxane. J. Am. Chem. Soc. 1960, 82, 6194–6195. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Takada, K.; Matsumoto, H. Synthesis of Ladder and Cage Silsesquioxanes from 1,2,3,4-Tetrahydroxycyclotetrasiloxane. Bull. Chem. Soc. Jpn. 2000, 73, 215–220. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, H. Pentacyclic Laddersiloxane. J. Am. Chem. Soc. 2002, 124, 1574–1575. [Google Scholar] [CrossRef] [PubMed]
- Suyama, K.; Gunji, T.; Arimitsu, K.; Abe, Y. Synthesis and Structure of Ladder Oligosilsesquioxanes: Tricyclic Ladder Oligomethylsilsesquioxanes. Organometallics 2006, 25, 5587–5593. [Google Scholar] [CrossRef]
- Unno, M.; Matsumoto, T.; Matsumoto, H. Synthesis of laddersiloxanes by novel stereocontrolled approach. J. Organomet. Chem. 2007, 692, 307–312. [Google Scholar] [CrossRef]
- Seki, H.; Abe, Y.; Gunji, T. Stereochemistry of the reaction of cis, trans, cis-2,4,6,8-tetraisocyanato-2,4,6,8-tetramethylcyclotetrasiloxane with triphenylsilanol and 1,1,3,3-tetraphenyldisiloxane-1,3-diol. J. Organomet. Chem. 2011, 696, 846–851. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Unno, M. Synthesis and Properties of Phenylsilsesquioxanes with Ladder and Double-Decker Structures. Organometallics 2014, 33, 4148–4151. [Google Scholar] [CrossRef]
- Sugiyama, T.; Shiba, H.; Yoshikawa, M.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of Polycyclic and Cage Siloxanes by Hydrolysis and Intramolecular Condensation of Alkoxysilylated Cyclosiloxanes. Chem. Eur. J. 2019, 25, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Janus ring siloxane: Versatile precursor of extended Janus ring and tricyclic laddersiloxanes. Dalton Trans. 2020, 49, 13533–13537. [Google Scholar] [CrossRef]
- Chaiprasert, T.; Liu, Y.; Intaraprecha, P.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Tricyclic Laddersiloxane with Various Ring Sizes (Bat Siloxane). Macromol. Rapid Commun. 2021, 42, 2000608. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Tanaka, R.; Tanaka, S.; Takeuchi, T.; Kyushin, S.; Matsumoto, H. Oligocyclic Ladder Polysiloxanes: Alternative Synthesis by Oxidation. Organometallics 2005, 24, 765–768. [Google Scholar] [CrossRef]
- Unno, M.; Chang, S.; Matsumoto, H. cis-trans-cis-Tetrabromotetramethylcyclotetrasiloxane: A Versatile Precursor of Ladder Silsesquioxanes. Bull. Chem. Soc. Jpn. 2005, 78, 1105–1109. [Google Scholar] [CrossRef]
- Liu, Y.; Onodera, K.; Takeda, N.; Ouali, A.; Unno, M. Synthesis and Characterizatioin of Functionalizable Silsesquioxanes with Ladder-type Structures. Organometallics 2019, 38, 4373–4376. [Google Scholar] [CrossRef]
- Liu, Y.; Endo, A.; Zhang, P.; Takizawa, A.; Takeda, N.; Ouali, A.; Unno, M. Synthesis, Characterization, and Reaction of Divinyl-substituted Laddersiloxanes. Silicon 2022, 14, 2723–2730. [Google Scholar] [CrossRef]
- Liu, Y.; Katano, M.; Yingsukkamol, P.; Takeda, N.; Unno, M.; Ouali, A. Tricyclic 6-8-6 laddersiloxanes derived from all-cis-tetravinylcyclotetrasiloxanolate: Synthesis, characterization and reactivity. J. Organomet. Chem. 2022, 959, 122213. [Google Scholar] [CrossRef]
- Pang, X.; Wan, C.; Wang, M.; Lin, Z. Strictly Biphasic Soft and Hard Janus Structures: Synthesis, Properties, and Applications. Angew. Chem. Int. Ed. 2014, 53, 5524–5538. [Google Scholar] [CrossRef]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus particles for biological imaging and sensing. Analyst 2016, 141, 3526–3539. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.-W.; Noor, N.; Zheng, Z. Graphene-based two-dimensional Janus materials. NPG Asia Mater. 2018, 10, 217–237. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Huang, J.; Guo, Z. Anisotropic Janus materials: From micro-/nanostructures to applications. Nanoscale 2021, 13, 18839–18864. [Google Scholar] [CrossRef]
- Liu, Y.; Kigure, M.; Okawa, R.; Takeda, N.; Unno, M.; Ouali, A. Synthesis and characterization of tetrathiol-substituted double-decker or ladder silsesquioxane nano-cores. Dalton Trans. 2021, 50, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Sun, Z.; Ansari, R.; Liu, Y.; Endo, A.; Unno, M.; Ouali, A.; Mahbub, S.; Furgal, J.C.; Yodsin, N.; et al. Conjugated Copolymers That Shouldn’t Be. Angew. Chem. Int. Ed. 2021, 60, 11115–11119. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tokuda, M.; Takeda, N.; Ouali, A.; Unno, M. New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and Reactivity. Molecules 2023, 28, 5699. https://doi.org/10.3390/molecules28155699
Liu Y, Tokuda M, Takeda N, Ouali A, Unno M. New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and Reactivity. Molecules. 2023; 28(15):5699. https://doi.org/10.3390/molecules28155699
Chicago/Turabian StyleLiu, Yujia, Midori Tokuda, Nobuhiro Takeda, Armelle Ouali, and Masafumi Unno. 2023. "New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and Reactivity" Molecules 28, no. 15: 5699. https://doi.org/10.3390/molecules28155699
APA StyleLiu, Y., Tokuda, M., Takeda, N., Ouali, A., & Unno, M. (2023). New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and Reactivity. Molecules, 28(15), 5699. https://doi.org/10.3390/molecules28155699