Development and Validation of a New UFLC–MS/MS Method for the Detection of Organophosphate Pesticide Metabolites in Urine
Abstract
:1. Introduction
2. Results and Discussion
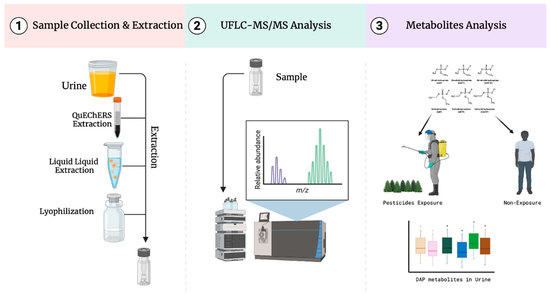
2.1. Sample Preparation
2.2. Method Validation
2.2.1. Selectivity
2.2.2. Linearity
2.2.3. LOQ and LOQ
2.2.4. Recovery
2.2.5. Precision and Accuracy
2.2.6. Matrix Effect
2.2.7. Stability
2.3. Method Application:
2.4. Discussion
3. Methods and Materials
3.1. Chemicals
3.2. Preparation of Standards and Calibration Standard
3.3. Sample Collection and Preparation
3.4. UFLC–MS/MS Conditions
3.5. Method Validation
3.5.1. Selectivity
3.5.2. Linearity
3.5.3. Recovery
3.5.4. LowerLimit of Detection and Quantification
3.5.5. Precision and Accuracy
3.5.6. Matrix Effects
3.5.7. Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Kaushik, G.; Villarreal-Chiu, J.F. Scenario of organophosphate pollution and toxicity in India: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 9480–9491. [Google Scholar] [CrossRef] [PubMed]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public. Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Eskenazi, B.; Harley, K.; Bradman, A.; Weltzien, E.; Jewell, N.P.; Barr, D.B.; Furlong, C.E.; Holland, N.T. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ. Health Perspect. 2004, 112, 1116–1124. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K. Pesticide exposure—Indian scene. Toxicology 2004, 198, 83–90. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.X.; Terry, A.V., Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B. Acetylcholinesterase—Apoptosis induction, role in neurological diseases and leukemia. Postep. Biochem. 2005, 51, 154–161. [Google Scholar]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. BioMed Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef] [Green Version]
- Kisby, G.E.; Muniz, J.F.; Scherer, J.; Lasarev, M.R.; Koshy, M.; Kow, Y.W.; McCauley, L. Oxidative stress and DNA damage in agricultural workers. J. Agromed. 2009, 14, 206–214. [Google Scholar] [CrossRef]
- Ledda, C.; Cannizzaro, E.; Cinà, D.; Filetti, V.; Vitale, E.; Paravizzini, G.; Di Naso, C.; Iavicoli, I.; Rapisarda, V. Oxidative stress and DNA damage in agricultural workers after exposure to pesticides. J. Occup. Med. Toxicol. 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Rothlein, J.; Rohlman, D.; Lasarev, M.; Phillips, J.; Muniz, J.; McCauley, L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and non-agricultural Hispanic workers. Environ. Health Perspect. 2006, 114, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Han, S.; Liang, D.; Shi, X.; Wang, F.; Liu, W.; Zhang, L.; Chen, L.; Gu, Y.; Tian, Y. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: A birth cohort study in Shenyang, China. PLoS ONE 2014, 9, e88491. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Garduño, C.; Lacasaña, M.; Blanco-Muñoz, J.; Rodríguez-Barranco, M.; Hernández, A.F.; Bassol, S.; González-Alzaga, B.; Cebrián, M.E. Changes in male hormone profile after occupational organophosphate exposure. A longitudinal study. Toxicology 2013, 307, 55–65. [Google Scholar] [CrossRef]
- Barr, D.B.; Bravo, R.; Weerasekera, G.; Caltabiano, L.M.; Whitehead, R.D., Jr.; Olsson, A.O.; Caudill, S.P.; Schober, S.E.; Pirkle, J.L.; Sampson, E.J.; et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health Perspect. 2004, 112, 186–200. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Barr, D.B.; Pearson, M.A.; Waller, L.A. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ. Health Perspect. 2008, 116, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Sinha, S.N.; Rajendra, S.; Sharma, K. Assessing farmer’s exposure to pesticides and the risk for non-communicable diseases: A biomonitoring study. Sci. Total Environ. 2023, 891, 164429. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Chevrier, J.; Harley, K.G.; Kogut, K.; Vedar, M.; Calderon, N.; Trujillo, C.; Johnson, C.; Bradman, A.; Barr, D.B.; et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 2011, 119, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, E.; Rose, R.L. Organophosphorus chemicals: Potent inhibitors of the human metabolism of steroid hormones and xenobiotics. Drug Metab. Rev. 2006, 38, 149–162. [Google Scholar] [CrossRef]
- Blanco-Muñoz, J.; Morales, M.M.; Lacasaña, M.; Aguilar-Garduño, C.; Bassol, S.; Cebrián, M.E. Exposure to organophosphate pesticides and male hormone profile in floriculturist of the state of Morelos, Mexico. Hum. Reprod. 2010, 25, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Koutros, S.; Beane Freeman, L.E.; Lubin, J.H.; Heltshe, S.L.; Andreotti, G.; Barry, K.H.; DellaValle, C.T.; Hoppin, J.A.; Sandler, D.P.; Lynch, C.F.; et al. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am. J. Epidemiol. 2013, 177, 59–74. [Google Scholar] [CrossRef]
- Benka-Coker, W.O.; Loftus, C.; Karr, C.; Magzamen, S. Characterizing the joint effects of pesticide exposure and criteria ambient air pollutants on pediatric asthma morbidity in an agricultural community. Environ. Epidemiol. 2019, 3, e046. [Google Scholar] [CrossRef] [PubMed]
- Dalsager, L.; Christensen, L.E.; Kongsholm, M.G.; Kyhl, H.B.; Nielsen, F.; Schoeters, G.; Jensen, T.K.; Andersen, H.R. Associations of maternal exposure to organophosphate and pyrethroid insecticides and the herbicide 2, 4-D with birth outcomes and anogenital distance at 3 months in the Odense Child Cohort. Reprod. Toxicol. 2018, 76, 53–62. [Google Scholar] [CrossRef]
- Tang, J.; ROSE, R.L.; Chambers, J.E. Metabolism of organophosphorus and carbamate pesticides. In Toxicology of Organophosphate & Carbamate Compounds; Elsevier: Amsterdam, The Netherlands, 2006; pp. 127–143. [Google Scholar]
- Barr, D.B.; Needham, L.L.J. Analytical methods for biological monitoring of exposure to pesticides: A review. J. Chromatogr. B 2002, 778, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Duggan, A.; Charnley, G.; Chen, W.; Chukwudebe, A.; Hawk, R.; Krieger, R.I.; Ross, J.; Yarborough, C. Di-alkyl phosphate biomonitoring data: Assessing cumulative exposure to organophosphate pesticides. Regul. Toxicol. Pharmacol. RTP 2003, 37, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Sheldon, L.S.; Croghan, C.W.; Jones, P.A.; Robertson, G.L.; Chuang, J.C.; Wilson, N.K.; Lyu, C.W. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Prapamontol, T.; Sutan, K.; Laoyang, S.; Hongsibsong, S.; Lee, G.; Yano, Y.; Hunter, R.E.; Ryan, P.B.; Barr, D.B.; Panuwet, P. Cross validation of gas chromatography-flame photometric detection and gas chromatography-mass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. Int. J. Hyg. Environ. Health 2014, 217, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Margariti, M.G.; Tsakalof, A.K.; Tsatsakis, A.M. Analytical methods of biological monitoring for exposure to pesticides: Recent update. Ther. Drug Monit. 2007, 29, 150–163. [Google Scholar] [CrossRef]
- Oglobline, A.N.; Elimelakh, H.; Tattam, B.; Geyer, R.; O’Donnell, G.E.; Holder, G. Negative ion chemical ionization GC/MS-MS analysis of dialkylphosphate metabolites of organophosphate pesticides in urine of non-occupationally exposed subjects. Analyst 2001, 126, 1037–1041. [Google Scholar] [CrossRef]
- Cequier, E.; Marcé, R.M.; Becher, G.; Thomsen, C. A high-throughput method for determination of metabolites of organophosphate flame retardants in urine by ultra performance liquid chromatography-high resolution mass spectrometry. Anal. Chim. Acta 2014, 845, 98–104. [Google Scholar] [CrossRef]
- Fernández, S.F.; Pastor, A.; Yusà, V.; Montesinos, L.; Pardo, O. Development of a novel methodology for determination of dialkyl phosphates in human urine using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1130–1131, 121810. [Google Scholar] [CrossRef]
- Stefanelli, P.; Barbini, D.A.; Girolimetti, S. Pesticides and their metabolites in human urine: Development of multi-analyte method by LC-MS/MS and GC-MS/MS. J. Environ. Sci. Health Part. B Pestic. Food Contam. Agric. Wastes 2021, 56, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Jamin, E.L.; Bonvallot, N.; Tremblay-Franco, M.; Cravedi, J.P.; Chevrier, C.; Cordier, S.; Debrauwer, L. Untargeted profiling of pesticide metabolites by LC-HRMS: An exposomics tool for human exposure evaluation. Anal. Bioanal. Chem. 2014, 406, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Dualde, P.; Yusà, V.; Coscollà, C. Retrospective analysis of pesticide metabolites in urine using liquid chromatography coupled to high-resolution mass spectrometry. Talanta 2016, 160, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Odetokun, M.S.; Montesano, M.A.; Weerasekera, G.; Whitehead, R.D., Jr.; Needham, L.L.; Barr, D.B. Quantification of dialkylphosphate metabolites of organophosphorus insecticides in human urine using 96-well plate sample preparation and high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2567–2574. [Google Scholar] [CrossRef]
- Davis, M.D.; Wade, E.L.; Restrepo, P.R.; Roman-Esteva, W.; Bravo, R.; Kuklenyik, P.; Calafat, A.M. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 929, 18–26. [Google Scholar] [CrossRef]
- Sinha, S.N.; Banda, V.R. Correlation of pesticide exposure from dietary intake and bio-monitoring: The different sex and socio-economic study of children. Ecotoxicol. Environ. Saf. 2018, 162, 170–177. [Google Scholar] [CrossRef]
- Dulaurent, S.; Saint-Marcoux, F.; Marquet, P.; Lachâtre, G. Simultaneous determination of six dialkylphosphates in urine by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 831, 223–229. [Google Scholar] [CrossRef]
- Reemtsma, T.; Lingott, J.; Roegler, S. Determination of 14 monoalkyl phosphates, dialkyl phosphates and dialkyl thiophosphates by LC-MS/MS in human urinary samples. Sci. Total Environ. 2011, 409, 1990–1993. [Google Scholar] [CrossRef]
- Sinha, S.N.; Reddy, B.V.; Vasudev, K.; Rao, M.V.V.; Ahmed, M.N.; Ashu, S.; Kumari, A.; Bhatnagar, V. Analysis of dialkyl urine metabolites of organophosphate pesticides by a liquid chromatography mass spectrometry technique. Anal. Methods 2014, 6, 1825–1834. [Google Scholar] [CrossRef]
- Ueyama, J.; Saito, I.; Takaishi, A.; Nomura, H.; Inoue, M.; Osaka, A.; Sugiura, Y.; Hayashi, Y.; Wakusawa, S.; Ogi, H.; et al. A revised method for determination of dialkylphosphate levels in human urine by solid-phase extraction and liquid chromatography with tandem mass spectrometry: Application to human urine samples from Japanese children. Environ. Health Prev. Med. 2014, 19, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Oya, N.; Ito, Y.; Hioki, K.; Asai, Y.; Aoi, A.; Sugiura, Y.; Ueyama, J.; Oguri, T.; Kato, S.; Ebara, T.; et al. Quantitative analysis of organophosphate insecticide metabolites in urine extracted from disposable diapers of toddlers in Japan. Int. J. Hyg. Environ. Health 2017, 220, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, R.R.; Tsai, P.C.; Lin, P.D.; Wu, M.T.; Ponnusamy, V.K. Rapid and sensitive analytical procedure for biomonitoring of organophosphate pesticide metabolites in human urine samples using a vortex-assisted salt-induced liquid-liquid microextraction technique coupled with ultra-high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2020, 34 (Suppl. 1), e8565. [Google Scholar] [CrossRef]
- Lu, Q.; Lin, N.; Cheng, X.; Yuan, T.; Zhang, Y.; Gao, Y.; Xia, Y.; Ma, Y.; Tian, Y. Simultaneous determination of 16 urinary metabolites of organophosphate flame retardants and organophosphate pesticides by solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry. Chemosphere 2022, 300, 134585. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, I.; Bora, S.; Adole, P.S.; Thyagaraju, C.; Nachiappa Ganesh, R. Assessment of Organophosphate Pesticides Exposure in Men with Idiopathic Abnormal Semen Analysis: A Cross-Sectional Pilot Study. Int. J. Fertil. Steril. 2021, 15, 219–225. [Google Scholar] [CrossRef]
- SANTE. Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post-Approval Control and Monitoring Purposes. 2023. Available online: https://food.ec.europa.eu/system/files/2023-04/pesticides_mrl_guidelines_2020-12830.pdf (accessed on 30 June 2023).
- Kumar, D.; Sinha, S.N.; Ungarala, R.; Mungamuri, S.K.; Kasturi, V. A simple and sensitive liquid chromatography–tandem mass spectrometry method for quantification of multi-residual pesticides in blood. Sep. Sci. Plus 2022, 5, 193–206. [Google Scholar] [CrossRef]




| Metabolites | Calibration Range (ng/mL) | R2 | SLOP | LOD ng/mL | LOQ ng/mL |
|---|---|---|---|---|---|
| DEP | 0.1–200 | 0.9997 | 9729.73 | 0.0201 | 0.0609 |
| DETP | 0.1–200 | 0.9992 | 8704.87 | 0.0323 | 0.0969 |
| DEDTP | 0.1–200 | 0.9964 | 19,603.50 | 0.0697 | 0.2112 |
| DMP | 0.1–200 | 0.9997 | 566.00 | 0.0207 | 0.0626 |
| DMTP | 0.1–200 | 0.9983 | 2196.01 | 0.0488 | 0.1479 |
| DMDTP | 0.1–200 | 0.9988 | 1365.28 | 0.0406 | 0.1229 |
| Metabolites | Concentration Added (ng/mL) | Inter-Day (n = 5) | Intra-Day (n = 15) | ||
|---|---|---|---|---|---|
| Accuracy (%) ± SD | Precision (RSD %) | Accuracy (%) ± SD | Precision (RSD%) | ||
| DEP | 0.5 | 95.00 ± 1.87 | 1.97 | 86.04 ± 7.57 | 8.80% |
| 25 | 100.46 ± 2.09 | 2.08 | 97.60 ± 3.23 | 3.31% | |
| 200 | 96.58 ± 0.86 | 0.89% | 95.41 ± 1.34 | 1.40% | |
| DETP | 0.5 | 94.13 ± 0.59 | 0.62 | 89.76 ± 9.27 | 10.33 |
| 25 | 98.10 ± 1.78 | 1.82 | 96.00 ± 2.49 | 2.60 | |
| 200 | 98.17 ± 1.46 | 1.48 | 96.99 ± 1.73 | 1.79 | |
| DMDTP | 0.5 | 93.18 ± 3.73 | 4.00 | 90.51 ± 5.75 | 6.35 |
| 25 | 100.98 ± 1.55 | 1.54 | 97.56 ± 3.53 | 3.62 | |
| 200 | 95.96 ± 1.52 | 1.58 | 96.85 ± 1.57 | 1.63 | |
| DMP | 0.5 | 99.67 ± 2.84 | 2.85 | 89.76 ± 9.79 | 10.91 |
| 25 | 100.98 ± 3.18 | 3.15 | 97.67 ± 4.25 | 4.35 | |
| 200 | 96.53 ± 1.48 | 1.53 | 94.60 ± 2.68 | 2.84 | |
| DMTP | 0.5 | 98.43 ± 1.31 | 1.33 | 93.43 ± 10.60 | 11.33 |
| 25 | 96.95 ± 0.80 | 0.82 | 96.95 ± 2.49 | 2.60 | |
| 200 | 92.29 ± 0.07 | 0.80 | 92.29 ± 0.74 | 0.80 | |
| DMDTP | 0.5 | 89.07 ± 4.86 | 5.46 | 88.90 ± 8.07 | 9.07 |
| 25 | 98.47 ± 0.62 | 0.63 | 97.82 ± 1.98 | 2.03 | |
| 200 | 87.47 ± 1.94 | 2.22 | 89.04 ± 2.21 | 2.48 | |
| Metabolites | Concentration ng/mL Mean ± Sd Exposed | Concentration ng/mL Mean ± Sd Non-Exposed |
|---|---|---|
| DEP | 13.04 ± 5.93 | 11.69 ± 4.97 |
| DETP | 1.25 ± 2.24 | 1.29 ± 1.51 |
| DEDTP | 0.58 ± 0.43 | 0.74 ± 0.62 |
| DMP | 16.88 ± 54.33 | 8.95 ± 4.41 |
| DMTP | 20.83 ± 21.60 | 18.19 ± 12.82 |
| DMDTP | 4.37 ± 7.27 | 2.84 ± 2.48 |
| Total DAP | 56.37 ± 59.69 | 43.71 ± 15.14 |
| Study | Extraction Method | Run Time (Minutes) | Solvent Volume (mL) | Sample Volume (mL) | Recovery (%) | LOD ng/mL |
|---|---|---|---|---|---|---|
| [40] | Liquid–liquid extraction | 22 | 11 | 5 | 13–99 | 0.2 |
| [37] | Solid-phase extraction | 10 | 5 | 0.600 | 40–98 | 0.04–1.5 |
| [41] | Liquid–liquid extraction | 28 | 5 | 3 | 81–122 | 1–6 |
| [42] | Liquid–liquid extraction (lyophilization) | 35 | 10 | 10 | 99–100 | 0.02–0.09 |
| [43] | Solid-phase extraction | 10 | 15 | 1 | 80–100 | 0.1–0.4 |
| [44] | Liquid–liquid extraction | 15 | 1 | 54–101 | 0.03–1.77 | |
| [33] | Liquid–liquid extraction | 20 | 8 | 4 | 70–112 | 0.50 |
| [45] | Liquid–liquid microextraction | 15 | 8 | 2 | 85.0–114 | 0.01–0.03 |
| [46] | Solid-phase extraction | 10 | - | 0.200 | 73–127 | 0.03–1.129 |
| This study | Liquid–liquid extraction | 6 | 2 | 0.200 | 93–102 | 0.02–0.06 |
| Metabolites | Parent Ions (m/z) | Product Ions (m/z) | DP | CE | CXP | RT |
|---|---|---|---|---|---|---|
| DEP | 152.9 | 78.9/125 | −47 | −26 | −1.5 | 2.44 |
| DETP | 168.8 | 140.8/95 | −54 | −18 | −9.7 | 1.97 |
| DEDTP | 184.7 | 110.8/157 | −54 | −29 | −3.3 | 1.92 |
| DMP | 125 | 62.8/110 | −68 | −4 | −2 | 2.38 |
| DMTP | 140.8 | 125.8/96 | −61 | −19 | −1 | 1.96 |
| DMDTP | 156.7 | 112/142 | −56 | −23 | −10 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Sinha, S.N.; Vasudev, K. Development and Validation of a New UFLC–MS/MS Method for the Detection of Organophosphate Pesticide Metabolites in Urine. Molecules 2023, 28, 5800. https://doi.org/10.3390/molecules28155800
Kumar D, Sinha SN, Vasudev K. Development and Validation of a New UFLC–MS/MS Method for the Detection of Organophosphate Pesticide Metabolites in Urine. Molecules. 2023; 28(15):5800. https://doi.org/10.3390/molecules28155800
Chicago/Turabian StyleKumar, Dileshwar, Sukesh Narayan Sinha, and Kasturi Vasudev. 2023. "Development and Validation of a New UFLC–MS/MS Method for the Detection of Organophosphate Pesticide Metabolites in Urine" Molecules 28, no. 15: 5800. https://doi.org/10.3390/molecules28155800
APA StyleKumar, D., Sinha, S. N., & Vasudev, K. (2023). Development and Validation of a New UFLC–MS/MS Method for the Detection of Organophosphate Pesticide Metabolites in Urine. Molecules, 28(15), 5800. https://doi.org/10.3390/molecules28155800