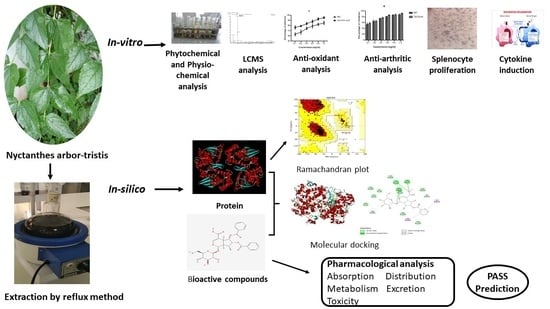
In Vitro and In Silico Anti-Rheumatic Arthritis Activity of Nyctanthes arbor-tristis
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Screening
2.1.1. Qualitative Phytochemical Screening
2.1.2. Total Flavonoids Content and Total Phenol Content
2.1.3. LCMS Analysis
2.2. In Vitro Anti-oxidant Activities
2.3. In Vitro Anti-Arthritic Activities
2.4. Effect of NAT Leaves on Splenocytes Proliferation
2.5. Effect of Nyctanthes Arbor-Tristis on Cytokines (TNF-α and IL-10) Induction
2.6. In Silico Studies
2.6.1. Preparation of Protein
2.6.2. Preparation of Ligands
2.6.3. Ramachandran Plot
2.6.4. Interactions between Bioactive Compounds (Ligands) and Proteins
2.6.5. Pharmacokinetic and Bioactivity Properties
2.6.6. Prediction of Activity Spectra for Substances (Pass) Prediction Study
3. Discussion
4. Materials and Methods
4.1. Sample Collection, Authentication, and Extraction
4.2. Preliminary Phytochemical Analysis
4.3. Quantitative Estimation of Total Phenols and Total Flavonoid
4.4. Liquid Chromatography Parameters
Mass Spectrometry Parameters
4.5. In Vitro Scavenging Potential Assays
4.5.1. DPPH Free Radical Scavenging Activity
4.5.2. Scavenging of Hydrogen Peroxide
4.6. In Vitro Anti-Arthritic Activities
4.6.1. Protein Denaturation Inhibition Assay
4.6.2. Membrane-Stabilization Assay
4.7. Splenocyte Proliferation Assay
4.8. In Vitro Determination of NAT Extract on Cytokine Production
4.9. In Silico Analysis
4.9.1. Preparation of Protein
4.9.2. Preparation of Ligand and Receptor Grid Generation
4.9.3. Pharmacology Analysis and Preclinical Trials
4.9.4. PASS (Prediction of Activity Spectra for Substances) Prediction Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Prasad, P.; Verma, S.; Ganguly, N.K.; Chaturvedi, V.; Mittal, S.A. Rheumatoid arthritis: Advances in treatment strategies. Mol. Cell. Biochem. 2023, 478, 69–88. [Google Scholar] [CrossRef]
- Garg, R.; Garg, A. The Research Trends and Scientometric Assessment of Rheumatoid Arthritis in India During 2016–2021. Curr. Rheumatol. Rev. 2023, 19, 26–35. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Wei, Z.F.; Jiao, X.L.; Wang, T.; Lu, Q.; Xia, Y.F.; Wang, Z.T.; Guo, Q.L.; Chou, G.X.; Dai, Y. Norisoboldine alleviates joint destruction in rats with adjuvant-induced arthritis by reducing RANKL, IL-6, PGE2, and MMP-13 expression. Acta Pharmacol. 2013, 34, 403–413. [Google Scholar] [CrossRef]
- Shen, L.; Wang, P.; Guo, J.; Du, G. Anti-arthritic activity of ethanol i of Fagopyrum cymosum with adjuvant-induced arthritis in rats. Pharm. Biol. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.H.; Lee, H.T.; Lee, J.U.; Son, J.Y.; Shin, W.; Heo, Y.S. Structural biology of the TNFα antagonists used in the treatment of rheumatoid arthritis. Int. J. Mol. Sci. 2018, 19, 768. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, V.; Gupta, P.; Singh, S. Investigation of antiarthritic potential of Plumeria alba L. leaves in acute and chronic models of arthritis. Biomed. Res. Int. 2014, 2014, 474616. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, V.; Gupta, P.K.; Singh, S. Anti-arthritic activity of Barleria prionitis Linn. leaves in acute and chronic models in Sprague Dawley rats. Bull. Fac. Pharm. 2014, 52, 199–209. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Suroowan, S.; Mahomoodally, F. Herbal products for common auto-inflammatory disorders—Novel approaches. Comb. Chem. High Throughput Screen. 2018, 21, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, R.T.; Minj, S.; Singh, V. Phytochemical chemical characters of Nyctanthes arbor-tristis Linn.: A promising medicinal plant. J. Med. Plant Res. 2019, 7, 141–143. [Google Scholar]
- Shrivastava, R.; Bharadwaj, A.K. Nyctanthes arbortristis an Important Medicinal Plant of Madhya Pradesh State—A Review. Pharm. Biosci. J. 2018, 6, 10–15. [Google Scholar] [CrossRef]
- Venkataraman, S.; Harinya, S.; Chidiuto, D.B.; Raja, R.R. Phytochemical Constituents and Pharmacological activities of Nyctanthes arbor-tristis. Res J Pharm Technol. 2019, 12, 4639–4643. [Google Scholar] [CrossRef]
- Bhalakiya, H.; Modi, N.R. Traditional medicinal uses, phytochemical profile and pharmacological activities of Nyctanthes arbortris. RJLBPCS 2019, 5, 1003–1023. [Google Scholar]
- Singh, J.; Singh, A.P.; Singh, A.P. Nyctanthes arbor-tristis: A comprehensive review. World J. Curr. Med. Pharm. Res. 2021, 3, 74–78. [Google Scholar] [CrossRef]
- Shubha, P.; Namratha, K.; Aparna, H.S.; Ashok, N.R.; Mustak, M.S.; Chatterjee, J.; Byrappa, K. Facile green reduction of graphene oxide using Ocimum sanctum hydroalcoholic extract and evaluation of its cellular toxicity. Mater. Chem. Phys. 2017, 198, 66–72. [Google Scholar] [CrossRef]
- Emon, N.U.; Alam, S.; Rudra, S.; Riya, S.R.; Paul, A.; Hossen, S.M.; Ganguly, A. Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: In vivo, in vitro, and in silico approaches. Food Sci. Nutri. 2021, 9, 833–846. [Google Scholar] [CrossRef]
- Alajangi, H.K.; Kaur, M.; Sharma, A.; Rana, S.; Thakur, S.; Chatterjee, M.; Barnwal, R.P. Blood–brain barrier: Emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mole. Br. 2022, 15, 1–28. [Google Scholar] [CrossRef]
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clinic. Pharmac. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Faber, K.N.; Müller, M.; Jansen, P.L. Drug transport proteins in the liver. Adv. Drug. Deliv. Rev. 2003, 55, 107–124. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef]
- Rein, P.; Mueller, R.B. Treatment with biologicals in rheumatoid arthritis: An overview. Rheumatol Ther. 2017, 4, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Alan, L.; Miller, N.D. Antioxidant flavonoids: Structure, function and chemical usage. Alt. Med. Rev. 1996, 1, 103–111. [Google Scholar]
- Roger, M.F.; Wink, M. Alkaloids: Biochemistry, Ecology and Medicinal Applications; Plenum Press: New York, NY, USA, 1998; pp. 2–3. [Google Scholar]
- Mehta, R.; Sethiya, N.K.; Mehta, C.; Shah, G.B. Anti–arthritis activity of roots of Hemidesmus indicus R.Br. (Anantmul) in rats. Asian Pac. J. Trop. Med. 2012, 12, 130–135. [Google Scholar] [CrossRef]
- Umapathy, E.; Ndebia, E.J.; Meeme, A.; Adam, B.; Menziwa, P.; Chungag, B.N. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res. 2010, 4, 789–795. [Google Scholar]
- Volluri, S.S.; Bammidi, S.R.; Chippada, S.C.; Meena, V. In-vitro anti-arthritic activity of methanolic extract of Bacopa monniera. IJCEPR 2011, 2, 156–159. [Google Scholar]
- Held, J.; Mosheimer-Feistritzer, B.; Gruber, J.; Mur, E.; Weiss, G. Methotrexate therapy impacts on red cell distribution width and its predictive value for cardiovascular events in patients with rheumatoid. Arthritis 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Khandelwal, V.; Bhatia, A.K.; Goel, A.; Choudhary, P.; Goel, R. Studies of Anthocephalus cadamba leaf extract on haematological and biochemical parameters of albino rats. J. Chem. Pharm. Res. 2015, 7, 765–771. [Google Scholar]
- Khandelwal, V.; Choudhary, P.K.; Goel, A.; Bhatia, A.K.; Gururaj, K. Immunomodulatory activity of Neolamarckia cadamba (Roxb.) Bosser with reference to IL-2 induction. Int. J. Tradit. Knowlegde 2018, 17, 451–459. [Google Scholar]
- Owen, J.A.; Punt, J.; Stranford, S.A. Kuby Immunology, 7th ed.; WH Freeman & Company: New York, NY, USA, 2013; pp. 105–137. [Google Scholar]
- Popa, C.; Netea, M.G.; Van Riel, P.L.; Meer van der, J.W.; Stalenhoef, A.F. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function, and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Tasker, S.A.; Treanor, J.J.; Paxton, W.B.; Wallace, M.R. Efficacy of influenza vaccination in HIV-infected persons: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1999, 131, 430–433. [Google Scholar] [CrossRef]
- Chopra, A.; Lavin, P.; Patwardhan, B.; Chitre, D. Randomized double blind trial of an ayurvedic plant derived formulation for treatment of rheumatoid arthritis. J. Rheumatol. 2000, 27, 1365–1372. [Google Scholar]
- Ghasemi, J.B.; Azizeh, A.; Fereshteh, S. Molecular Docking Challenges and Limitations. In Applied Case Studies and Solutions in Molecular Docking-Based Drug Design; IGI Global: Hershey, PA, USA, 2016; pp. 56–80. [Google Scholar]
- Prashant, T.; Bimlesh, K.; Mandeep, K.; Gurpreet, K.; Harleen, K. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Kokate, C.K. Practical Pharmacognosy; Vallabh Prakashan: New Delhi, India, 2005; pp. 107–111. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods for Agricultural Sciences; Wiley Eastern Limited: New Delhi, India, 1992; pp. 6–7, 11–12. [Google Scholar]
- Tristantini, D.; Amalia, R. Quercetin concentration and total flavonoid content of anti-atherosclerotic herbs using aluminum chloride colorimetric assay. AIP Conf. Proc. 2019, 2193, 030012. [Google Scholar]
- Jeyadevi, R.; Ananth, D.A.; Sivasudha, T. Hepatoprotective and antioxidant activity of Ipomoea staphylina Linn. Clin. Phytosci. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. Hydrogen peroxide radical scavenging and total antioxidant activity of hawthorn. Chem. J 2012, 2, 9–12. [Google Scholar]
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, D.; Vimalan, M.; Potheher, I.V. Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their anti-bacterial, anti-arthritic, anti-oxidant and in vitro cytotoxicity activities. N. J. Chem. 2017, 41, 10347–10356. [Google Scholar] [CrossRef]
- Sharma, L.; Sharma, A.; Gupta, G.L. Standardization of a polyherbal preparation (POL–6) for treatment of oxidative, inflammatory and immune disorders. Int. J. Pharm. Pharm. Sci. 2016, 8, 129–134. [Google Scholar]
- Kumar, L.D.; Prathiviraj, R.; Selvakumar, M.; Guna, R.; Abbirami, E.; Sivasudha, T. HRLC-ESI-MS based identification of active small molecules from Cissus quadrangularis and likelihood of their action towards the primary targets of osteoarthritis. J. Mol. Struct. 2020, 1199, 127048. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Geronikaki, A.; Poroikov, V.; Hadjipavlou-Litina, D.; Filimonov, D.; Lagunin, A.; Mgonzo, R. Computer aided predicting the biological activity spectra and experimental testing of new thiazole derivatives. Quant. Struct. Act. Relatsh. 1999, 18, 16–25. [Google Scholar] [CrossRef]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef] [PubMed]





| S. No. | RT | Mol. Mass | ES+ | ES− | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| [M + H]+ | [M + Na]+ | [M − H]− | [M + Cl]− | [M + HCOO]− | [M + 2H]− | Compound Name | ||||
| 1 | 6.7 | 610 | 633 | 609 | 645 | Figure 1A,B | Unknown | |||
| 2 | 7.4 | 478 | 477 | 955 | Figure 1C | Calceolarioside A | ||||
| 3 | 10.4 | 510 | 511 | 533 | 545 | 555 | Figure 1D,E | Arborside C | ||
| 4 | 12.1 | 536 | 537 | 559 | 581 | Figure 1F,G | Carotenoid | |||
| 5 | 13.3 | 630 | 631 | 653 | 675 | Figure 1H,I | Unknown | |||
| 6 | 24.1 | 456 | 455 | Figure 1J | Nyctanthic Acid/ Oleanolic Acid | |||||
| S. No. | Conc. of Con-A in µg/mL | Conc. Of NAT Extract (µg/mL) | Mean Absorbance ± SEM at 540 nm | Stimulation Index (%) |
|---|---|---|---|---|
| 1. | 10 | Nil | 0.503 ± 0.006 | - |
| 2. | 10 | 50 | 0.689 ± 0.023 | 36.97 |
| 3. | 10 | 100 | 0.718 ± 0.011 | 42.74 |
| 4. | 10 | 250 | 0.783 ± 0.018 | 55.66 |
| 5. | 10 | 500 | 0.528 ± 0.012 | 4.97 |
| 6. | 10 | 1000 | 0.318 ± 0.017 | −36.77 |
| S. No. | Concentration | Mean Absorbance ± SEM of TNF-α | Mean Absorbance ± SEM of IL-10 | Stimulation Index (%) of TNF-α | Stimulation Index (%) of IL-10 |
|---|---|---|---|---|---|
| 1 | Control | 43.73 | 1358.12 | - | - |
| 2 | 50 | 64.76 | 1434.69 | 48.09 | 5.63 |
| 3 | 100 | 57.81 | 1593.91 | 32.19 | 17.36 |
| 4 | 250 | 53.95 | 2158.79 | 23.37 | 58.95 |
| 5 | 500 | 49.81 | 2330.74 | 13.9 | 71.61 |
| 6 | 1000 | 47.42 | 2235.84 | 8.43 | 64.62 |
| Properties | Arborside C | Calceolarioside A | Carotenoid | Nyctanthic Acid | Oleanoic Acid |
|---|---|---|---|---|---|
| Pgb-substrate | Yes | Yes | Yes | No | No |
| GI absorption (Gastrointestinal Absorption) | Low | Low | Low | Low | Low |
| BBB (Blood -Brain Barrier) | No | No | No | No | No |
| CYP450 1A2 inhibition | No | No | No | No | No |
| CYP450 3A4 inhibition | No | No | No | No | No |
| CYP450 2C9 inhibition | No | No | No | Yes | No |
| CYP450 2C19 inhibition | No | No | No | No | No |
| CYP450 2D6 inhibition | No | No | No | No | No |
| Skin permeation | −9.51 cm/s | −8.80 cm/s | −1.14 cm/s | −2.45 cm/s | −3.77 cm/s |
| Bioavailability Score | 0.11 | 0.17 | 0.17 | 0.85 | 0.85 |
| Synthetic accessibility | 6.14 | 5.20 | 5.82 | 5.73 | 6.08 |
| Phytoconstituents | Predicted LD50 | Predicted Toxicity Class | Pa | Pi | Activity |
|---|---|---|---|---|---|
| Arborside C | 2000 mg/kg | 4 | 0.798 | 0.007 | Anti-inflammatory |
| 0.738 | 0.012 | Immuno-suppressant | |||
| Calceolarioside A | 5000 mg/kg | 5 | 0.946 | 0.001 | Free radical scavenger |
| 0.716 | 0.04 | Anti-oxidant | |||
| Carotenoid | 4000 mg/kg | 5 | 0.746 | 0.011 | Immunosuppressant |
| Nyctanthic acid | 11,800 mg/kg | 2 | 0.772 | 0.009 | Anti-inflammatory |
| Oleanoic acid | 2000 mg/kg | 4 | 0.819 | 0.005 | Anti-inflammatory |
| 0.814 | 0.002 | Nitric oxide antagonist |
| S. No. | Time | Flow Rate (mL/min) | Solvent A (Acetonitrile) | Solvent B (0.1% Formic Acid in 95:5 v/v Water/Acetonitrile) |
|---|---|---|---|---|
| 1. | 0.1 | 0.25 | 5 | 95 |
| 2. | 1.0 | 0.25 | 5 | 95 |
| 3. | 10.0 | 0.25 | 30 | 70 |
| 4. | 14.0 | 0.25 | 60 | 40 |
| 5. | 16.0 | 0.25 | 60 | 40 |
| 6. | 24.0 | 0.25 | 80 | 20 |
| 7. | 32.0 | 0.25 | 80 | 20 |
| 8. | 35.0 | 0.25 | 5 | 95 |
| 9. | 40.0 | 0.25 | 5 | 95 |
| Source Temperature | 120 °C |
| Desolvation Temperature | 350 °C |
| Capillary | 3.5(kV) |
| Cone | 30V |
| Cone Gas Flow | 50 (L/h) |
| Desolvation Gas Flow | 950 (L/h) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Goel, A.; Lin, Z. In Vitro and In Silico Anti-Rheumatic Arthritis Activity of Nyctanthes arbor-tristis. Molecules 2023, 28, 6125. https://doi.org/10.3390/molecules28166125
Sharma A, Goel A, Lin Z. In Vitro and In Silico Anti-Rheumatic Arthritis Activity of Nyctanthes arbor-tristis. Molecules. 2023; 28(16):6125. https://doi.org/10.3390/molecules28166125
Chicago/Turabian StyleSharma, Ayushi, Anjana Goel, and Zhijian Lin. 2023. "In Vitro and In Silico Anti-Rheumatic Arthritis Activity of Nyctanthes arbor-tristis" Molecules 28, no. 16: 6125. https://doi.org/10.3390/molecules28166125
APA StyleSharma, A., Goel, A., & Lin, Z. (2023). In Vitro and In Silico Anti-Rheumatic Arthritis Activity of Nyctanthes arbor-tristis. Molecules, 28(16), 6125. https://doi.org/10.3390/molecules28166125