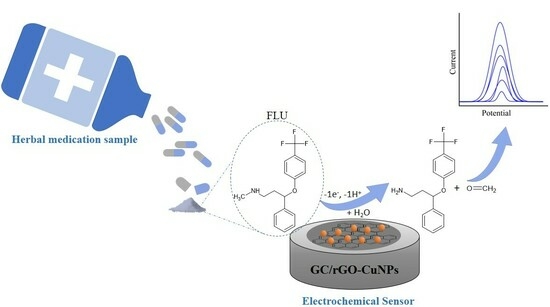
Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on rGO-CuNPs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Electrochemical Characterization of the Materials
2.2. Electrochemical Behavior of the Modified Electrodes
2.3. Fluoxetine Electrochemical Oxidation Processes
2.4. Evaluation of the Electrodes Modified with the Nanocomposites during the Fluoxetine Oxidation Process
2.5. Analysis of the Optimization Parameters for the Detection of Fluoxetine in Response to the GC/rGO-CuNPs Electrode
2.6. Analytical Curve
| Electrode | LOD (mol L−1) | Real Samples | Ref. |
|---|---|---|---|
| ZnO nanoparticles oriented MIP modified GCE | 2.67 × 10−12 | Tap water and spike serums | [26] |
| MIP 1 (itaconic acid monomer) modified GCE | 3.33 × 10−7 | Blood plasma | [27] |
| MIP (methacrylic acid monomer) modified CPE | 2.8 × 10−9 | Spiked plasma samples and fluoxetine capsules | [28] |
| BDD 2 electrode | 1.07 × 10−10 | Aqueous media | [39] |
| EGFET sensor 3 | 2.63 × 10−12 | Phosphate-buffer saline (PBS) | [43] |
| PVC/PEDOT-C14-modified electrode 4 | 3.5 × 10−8 | Tap and river water samples | [44] |
| BDD electrode | 2.90 × 10−7 | Thermogenic supplements, compounded drugs and weight loss herbal medicines | [42] |
| GC/rGO-CuNPs | 1.4 × 10−7 | Herbal medication | This work |
2.7. Determination of Fluoxetine in Herbal Medication
2.8. Fluoxetine Oxidation Mechanism
3. Materials and Methods
3.1. Instrumentation
3.2. Reagents and Solutions
3.3. Synthesis of the Materials rGO and rGO-CuNPs
3.4. Working Electrode Cleaning and Modification Steps
3.5. Preparation of the Plant-Derived Medication
3.6. Theoretical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araújo, L.S.; Coutinho, M.D.P.L.; Araújo-Morais, L.C.; Simeão SD, S.S.; Maciel, S.C. Prejudice towards obesity: Social representations in printed media. Arq. Bras. Psicol. 2018, 70, 69–85. [Google Scholar]
- Marks, R.; Foe, A.D.; Collett, J. The pursuit of wellness: Social media, body image and eating disorders. Child. Youth Serv. Rev. 2020, 119, 105659. [Google Scholar] [CrossRef]
- Grabe, S.; Ward, L.M.; Hyde, J.S. The role of the media in body image concerns among women: A meta-analysis of experimental and correlational studies. Psychol. Bull. 2008, 134, 460–476. [Google Scholar] [CrossRef]
- Groesz, L.M.; Levine, M.P.; Murnen, S.K. The effect of experimental presentation of thin media images on body satisfaction: A meta-analytic review. Int. J. Eat. Disord. 2002, 31, 1–16. [Google Scholar] [CrossRef]
- Homan, K.; McHugh, E.; Wells, D.; Watson, C.; King, C. The effect of viewing ultra-fit images on college women’s body dissatisfaction. Body Image 2012, 9, 50–56. [Google Scholar] [CrossRef]
- Tucci, S.; Peters, J. Media Influence on body satisfaction in female students. Psicothema 2008, 20, 521–524. [Google Scholar]
- Zambon, C.P.; Tiegs, L.M.R.; Campana, G.A.; da Silva Nunes, J. O uso de medicamentos fitoterápicos no processo de emagrecimento em acadêmicos do curso de farmácia da faculdade de Educação e Meio Ambiente—FAEMA. Rev. Cient. FAEMA 2018, 9, 500–506. [Google Scholar] [CrossRef]
- Verrengia, E.C.; Kinoshita, S.A.T.; Amadei, J.L. Medicamentos fitoterápicos no tratamento da obesidade. Uniciências 2015, 17, 53–58. [Google Scholar]
- Nakonieczny, L.; Watanabe, S.H.; Salin, D.N.O. Análise da presença de emagrecedores sintéticos em fitoterápicos comercializados nas farmácias e no mercado informal das cidades de União da Vitória–PR e Porto União–SC. Rev. Renov. 2019, 3, 101–132. [Google Scholar]
- Gnoatto, A.R.; Silva, S.C.S.; de Freitas, F.A.; de Almeida, M.T.R. Identification of anorexigen not declared in product marketed as phytotherapy. Braz. J. Health Rev. 2021, 4, 5385–5394. [Google Scholar] [CrossRef]
- De Carvalho, L.M.; Martini, M.; Moreira, A.P.; Garcia, S.C.; do Nascimento, P.C.; Bohrer, D. Determination of synthetic pharmaceuticals in phytotherapeutics by capillary zone electrophoresis with contactless conductivity detection (CZE-C4D). Microchem. J. 2010, 96, 114–119. [Google Scholar] [CrossRef]
- Calahan, J.; Howard, D.; Almalki, A.J.; Gupta, M.P.; Calderón, A.I. Chemical adulterants in herbal medicinal products: A review. Planta Med. 2016, 82, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Qu, J.; Luo, G.; Wang, Y. Rapid and reliable determination of illegal adulterant in herbal medicines and dietary supplements by LC/MS/MS. J. Pharm. Biomed. Anal. 2006, 40, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, F. Adulterated pharmaceutical chemicals in botanical dietary supplements: Novel screening approaches. Rev. Anal. Chem. 2017, 36, 20160032. [Google Scholar] [CrossRef]
- Ramos, D.L.O. Desenvolvimento de um Método Simples e de Baixo Custo Para Detecção de Fluoxetina em Produtos Emagrecedores; Monography; UFU: Uberlândia, Brazil, 2018. [Google Scholar]
- Da Silva, D.T.; Campos, C.A.M.; Vargas, T.G.; Ziulkoski, A.L.; Andrighetti, L.H.; Perassolo, M.S. Possíveis interações medicamentosas em pacientes polimedicados de Novo Hamburgo, RS, Brasil. Infarma Ciências Farm. 2018, 30, 21–29. [Google Scholar] [CrossRef]
- Lowinsohn, D.; Bertotti, M. Sensores eletroquímicos: Considerações sobre mecanismos de funcionamento e aplicações no monitoramento de espécies químicas em ambientes microscópicos. Rev. Quim. Nova 2006, 29, 1318–1325. [Google Scholar] [CrossRef]
- Scontri, M. Sensores Eletroquímicos à Base de Nanomateriais Carbonáceos e Catalisadores Biomiméticos para Determinação de Tetraciclina em Diversos Tipos de Amostras. Master’s Thesis, UNESP, Araraquara, Brazil, 2015. [Google Scholar]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Gomes, G.C.; da Silva, M.K.L.; Barreto, F.C.; Cesarino, I. Electrochemical Sensing Platform Based on Renewable Carbon Modified with Antimony Nanoparticles for Methylparaben Detection in Personal Care Products. Chemosensors 2023, 11, 141. [Google Scholar] [CrossRef]
- Barreto, F.C.; da Silva, M.K.L.; Cesarino, I. Copper Nanoparticles and Reduced Graphene Oxide as an Electrode Modifier for the Development of an Electrochemical Sensing Platform for Chloroquine Phosphate Determination. Nanomaterials 2023, 13, 1436. [Google Scholar] [CrossRef]
- Din, M.I.; Rehan, R. Synthesis, Characterization, and Applications of Copper Nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Trindade, C.M.; Silva, M.K.; Cesarino, I. Copper nanostructures anchored on renewable carbon as electrochemical platform for the detection of dopamine, fluoxetine and escitalopram. Sens. Actuators Rep. 2022, 4, 100107. [Google Scholar] [CrossRef]
- Çorman, M.E.; Cetinkaya, A.; Armutcu, C.; Uzun, L.; Ozkan, S.A. Designing of ZnO nanoparticles oriented interface imprinted electrochemical sensor for fluoxetine detection. Bioelectrochemestry 2023, 153, 108411. [Google Scholar] [CrossRef]
- Feroz, M.; Lopes, I.C.; Rehman, H.U.; Ata, S.; Vadgama, P. A novel molecular imprinted Polymer layer electrode for enhanced sensitivity electrochemical determination of the antidepressant fluoxetine. J. Electroanal. Chem. 2020, 878, 114693. [Google Scholar] [CrossRef]
- Alizadeh, T.; Azizi, S. Graphene/graphite paste electrode incorporated with molecularly imprinted polymer nanoparticles as a novel sensor for differential pulse voltammetry determination of fluoxetine. Biosens. Bioelectron. 2016, 81, 198–206. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H. Adulteration of Weight Loss Supplements by the Illegal Addition of Synthetic Pharmaceuticals. Molecules 2021, 26, 6903. [Google Scholar] [CrossRef]
- Foroughi, M.H.; Akhgari, M.; Jokar, F.; Zahra, M. Identification of undeclared active pharmaceutical ingredients in counterfeit hebal medicines used as opioid substituition therapy. Aust. J. Forensic Sci. 2017, 49, 720–729. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Velmurugan, M.; Chen, S.M.; Hwa, K.Y. Optimized electrochemical synthesis of copper nanoparticles decorated reduced graphene oxide: Application for enzymeless determination of glucose in human blood. J. Electroanal. Chem. 2017, 807, 128–136. [Google Scholar] [CrossRef]
- Moradi, P.; Hajjami, M.; Tahmasbi, B. Fabricated copper catalyst on biochar nanoparticles for the synthesis of tetrazoles as antimicrobial agents. Polyhedron 2020, 175, 114169. [Google Scholar] [CrossRef]
- Karuppannan, S.K.; Ramalingam, R.; Khalith, S.B.M.; Dowlath, M.J.H.; Raiyaan, G.I.D.; Arunachalam, K.D. Characterization, antibacterial and photocatalytic evaluation of green synthesized copper oxide nanoparticles. Biocatal. Agric. Biotechnol. 2021, 31, 101904. [Google Scholar] [CrossRef]
- Garcia, A.F.; Rollemberg, M.D.C. Voltammetric determination of glyphosate in natural waters with a copper electrode. Quím. Nova 2007, 30, 1592–1596. [Google Scholar]
- Pintado, S.; Montoya, M.R.; Rodríguez-Amaro, R.; Mayén, M.; Mellado, J.M.R. Electrochemical determination of glyphosate in waters using electrogenerated copper ions. Int. J. Electrochem. Sci. 2012, 7, 2523–2530. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct electrochemical determination of glyphosate at copper phthalocyanine/multiwalled carbon nanotube film electrodes. Electroanalysis 2010, 22, 1586–1591. [Google Scholar] [CrossRef]
- Lencastre, R.; Matos, C.; Garrido, J.; Borges, F.; Garrido, E. Voltammetric quantification of fluoxetine: Application to quality control and quality assurance processes. J. Food Drug Anal. 2006, 14, 242–246. [Google Scholar] [CrossRef]
- Garrido, E.M.; Garrido, J.; Calheiros, R.; Marques, M.P.M.; Borges, F. Fluoxetine and Norfluoxetine Revisited: New Insights into the Electrochemical and Spectroscopic Properties. J. Phys. Chem. A 2009, 113, 9934–9944. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, M.; Manea, F.; Pode, R. Electrochemical detection of fluoxetine using a boron-doped diamond electrode. Int. J. Pharm. Pharm. Sci. 2013, 5, 318–322. [Google Scholar]
- Da Silva, M.L.K.; Plana Simões, R.; Cesarino, I. Evaluation of reduced graphene oxide modified with antimony and copper nanoparticles for levofloxacin oxidation. Electroanalysis 2018, 30, 2066–2076. [Google Scholar] [CrossRef]
- Setznagl, S. Desenvolvimento e Caracterização de Dispositivo Eletroquímico Baseado em Nanopartículas de Cobre Suportadas Sobre Grafeno Para a Deteção do Herbicida Glifosato. Master’s Thesis, UNESP, Botucatu, Brazil, 2018. [Google Scholar]
- Ramos, D.L.O.; Freitas, J.M.; Munoz, R.A.A.; Richter, E.M. Simple and rapid voltammetric method for the detection of the synthetic adulterant fluoxetine in weight loss products. J. Electroanal. Chem. 2021, 882, 115028. [Google Scholar] [CrossRef]
- Shelbani, S.; Ionescu, A.M.; Norouzi, P. Highy sensitive detection of the antidepressant fluoxetine with and extended gate field effect transistor. IEEE Sens. J. 2022, 22, 9276–9288. [Google Scholar] [CrossRef]
- Izadyar, A.; Arachchige, D.R.; Cornwell, H.; Hersberger, J.C. Ion transfer stripping voltammetry for the detection of nanomolar levels of fluoxetine, citalopram, and sertraline in tap and river water samples. Sens. Actuators B Chem. 2016, 223, 226–233. [Google Scholar]
- Kim, H.J.; Lee, J.H.; Park, H.J.; Cho, S.H.; Cho, S.; Kim, W.S. Monitoing of 29 weight loss compounds in foods and dietary supplements by LC-MS/MS. Food Addit. Contam. Part A 2014, 31, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Silva, M.K.L.; Cesarino, I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors 2022, 10, 395. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; et al. Gaussian 09, Revision A.02. 1 January 2009. Available online: https://www.scienceopen.com/document?vid=6be7271f-f651-464b-aee6-ef20b0743b6b (accessed on 15 March 2018).
- Cesarino, I.; Simões, R.P.; Lavarda, F.C.; Batagin-Neto, A. Electrochemical oxidation of sulfamethazine on a glassy carbon electrode modified with graphene and gold nanoparticles. Electrochim. Acta 2016, 192, 8–14. [Google Scholar] [CrossRef]
- Donini, C.A.; da Silva, M.K.L.; Simões, R.P.; Cesarino, I. Reduced graphene oxide modified with silver nanoparticles for the electrochemical detection of estriol. J. Electroanal. Chem. 2018, 809, 67–73. [Google Scholar] [CrossRef]
- Cesarino, V.; Cesarino, I.; Moraes, F.C.; Machado, S.A.S.; Mascaro, L.H. Carbon nanotubes modified with SnO2 rods for levofloxacin detection. J. Braz. Chem. Soc. 2014, 25, 502–508. [Google Scholar]
- Nazario de Moraes, L.; Tommasini Grotto, R.M.; Targino Valente, G.; de Carvalho Sampaio, H.; Magro, A.J.; Fogaça, L.; Wolf, I.R.; Perahia, D.; Silva, G.F.; Simões, R.P. A novel molecular mechanism to explain mutations of the HCV protease associated with resistance against covalently bound inhibitors. Virus Res. 2019, 274, 197778. [Google Scholar] [CrossRef]
- Azevedo Neto, N.F.; Angelico, J.C.; da Silva Pelissari, M.R.; Camargo, L.P.; Simões, R.P.; Dall’Antonia, L.H.; Dias da Silva, J.H. Reactive sputtering deposition of Co3O4 films and an evaluation of its use as an electrochemical sensor for ascorbic acid. J. Mater. Sci. Mater. Electron. 2022, 33, 19678–19692. [Google Scholar] [CrossRef]
- Gasparini, P.; Philot, E.A.; Pantaleão, S.Q.; Torres-Bonfim, N.E.S.M.; Kliousoff, A.; Quiroz, R.C.N.; Perahia, D.; Simões, R.P.; Magro, A.J.; Scott, A.L. Unveiling mutation effects on the structural dynamics of the main protease from SARS-CoV-2 with hybrid simulation methods. J. Mol. Graph. Model. 2023, 121, 108443. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]










| Repetition | Fluoxetine (μmol L−1) a | Relative Errors b |
|---|---|---|
| 1 | 0.817 | 2.13 |
| 2 | 0.792 | −1.00 |
| 3 | 0.783 | −2.13 |
| Mean ± SD | 0.800 ± 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melaré, A.G.; Barreto, F.C.; Silva, M.K.L.; Simões, R.P.; Cesarino, I. Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on rGO-CuNPs. Molecules 2023, 28, 6361. https://doi.org/10.3390/molecules28176361
Melaré AG, Barreto FC, Silva MKL, Simões RP, Cesarino I. Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on rGO-CuNPs. Molecules. 2023; 28(17):6361. https://doi.org/10.3390/molecules28176361
Chicago/Turabian StyleMelaré, Aline Giuli, Francisco Contini Barreto, Martin Kassio Leme Silva, Rafael Plana Simões, and Ivana Cesarino. 2023. "Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on rGO-CuNPs" Molecules 28, no. 17: 6361. https://doi.org/10.3390/molecules28176361
APA StyleMelaré, A. G., Barreto, F. C., Silva, M. K. L., Simões, R. P., & Cesarino, I. (2023). Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on rGO-CuNPs. Molecules, 28(17), 6361. https://doi.org/10.3390/molecules28176361