Electrode Materials, Structural Design, and Storage Mechanisms in Hybrid Supercapacitors
Abstract
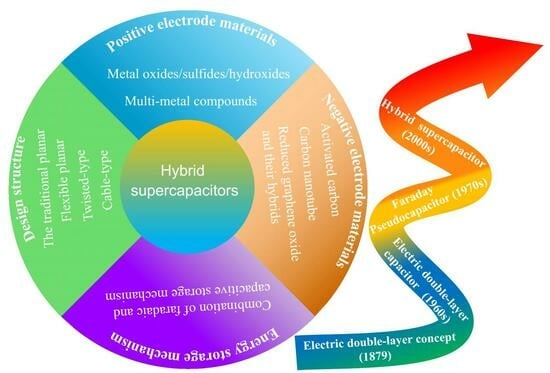
:1. Introduction
2. Recent Advances in Materials for Hybrid Supercapacitors
2.1. Positive Electrode Materials
2.1.1. Nickel Oxides/Hydroxides/Sulfides
2.1.2. Cobalt Oxides/Hydroxides
2.1.3. Multi-Metal Compounds
2.2. Negative Electrode Materials
2.2.1. Activated Carbon Materials
2.2.2. Carbon Nanotube Materials
2.2.3. Reduced Graphene Oxide Materials and Their Hybrids
3. Design Structures of Hybrid Supercapacitors
3.1. The Traditional Planar HSC Devices
3.2. The Flexible HSC Devices
3.3. The Twisted-Type HSC Devices
3.4. The Cable-Type HSC Devices
4. The Charge-Storage Mechanism of Hybrid Supercapacitors
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.C.; Deng, J.; Olivier, F. Breaking the strength barrier. Nat. Energy 2023, 23, 1264. [Google Scholar] [CrossRef]
- Wang, F.; Lee, J.Y.; Chen, L.; Zhang, G.Y.; He, S.J.; Han, J.Q.; Ahn, J.; Cheong, J.Y.; Jiang, S.H.; Kim, I.D. Inspired by Wood: Thick Electrodes for Supercapacitors. ACS Nano 2023, 17, 8866–8898. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.W.; Lei, T.; Lukatskaya, M.R.; Park, J.; Huang, Z.H.; Lee, M.; Shaw, L.; Chen, S.C.; Yakovenko, A.A.; Kulkarni, A.; et al. Robust and conductive two-dimensional metal-organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Li, K.D.; Zhao, T.; Wang, H.F.; Zhang, S.; Deng, C. From 1D nanotube arrays to 2D nanosheet networks on silver-coated textiles: New insights into the factors determining the performance of a core-shell hierarchical structure for wearable supercapacitors. J. Mater. Chem. A 2018, 6, 1561–1573. [Google Scholar] [CrossRef]
- Li, X.M.; Zheng, Q.W.; Li, C.M.; Liu, G.Q.; Yang, Q.Z.; Wang, Y.C.; Sun, P.C.; Tian, H.M.; Wang, C.H.; Chen, X.L.; et al. Bubble Up Induced Graphene Microspheres for Engineering Capacitive Energy Storage. Adv. Energy Mater. 2023, 13, 2203761. [Google Scholar] [CrossRef]
- Ma, H.Y.; Liang, J.; Qiu, J.; Li, J.; Ma, L.X.; Sheng, H.W.; Shao, M.J.; Wang, Q.; Li, F.F.; Fu, Y.J.; et al. A Biocompatible Supercapacitor Diode with Enhanced Rectification Capability toward Ion/Electron-Coupling Logic Operations. Adv. Mater. 2023, 35, 2301218. [Google Scholar] [CrossRef]
- Brousse, T.; Belanger, D.; Long, J.W. To Be or Not to Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from supercapacitor to battery behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Luo, X.Y.; Zheng, H.; Lai, W.D.; Yuan, P.; Li, S.W.; Li, D.; Chen, Y. Defect Engineering of Carbons for Energy Conversion and Storage Applications. Energy Environ. Mater. 2023, 6, e12402. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef]
- Dai, S.G.; Bai, Y.C.; Shen, W.X.; Zhang, S.; Hu, H.; Fu, J.W.; Wang, X.C.; Hu, C.G.; Liu, M.L. Core-shell structured Fe2O3@Fe3C@C nanochains and Ni-Co carbonate hydroxide hybridized microspheres for high-performance battery-type supercapacitor. J. Power Sources 2021, 482, 228915. [Google Scholar] [CrossRef]
- Dai, S.G.; Zhao, B.; Qu, C.; Chen, D.C.; Dang, D.; Song, B.; deGleea, B.M.; Fu, J.W.; Hu, C.G.; Wong, C.P.; et al. Controlled synthesis of three-phase NixSy/rGO nanoflake electrodes for hybrid supercapacitors with high energy and power density. Nano Energy 2017, 33, 522–531. [Google Scholar] [CrossRef]
- Zuo, W.H.; Li, R.Z.; Zhou, C.; Li, Y.Y.; Xia, J.L.; Liu, J.P. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.G.; Zhang, Z.F.; Xu, J.M.; Shen, W.X.; Zhang, Q.B.; Yang, X.G.; Xu, T.T.; Dang, D.; Hu, H.; Zhao, B.T.; et al. In situ Raman study of nickel bicarbonate for high-performance energy storage device. Nano Energy 2019, 64, 103919. [Google Scholar] [CrossRef]
- Huang, N.B.; Sun, Y.; Liu, S.; Wang, X.Y.; Zhang, J.J.; Guo, L.K.; Bi, J.P.; Sun, X.N. Microwave-Assisted Rational Designed CNT-Mn3O4/CoWO4 Hybrid Nanocomposites for High Performance Battery-Supercapacitor Hybrid Device. Small 2023, e2300696. [Google Scholar] [CrossRef]
- Soserov, L.; Marinova, D.; Koleva, V.; Stoyanova, A.; Stoyanova, R. Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors. Batteries 2022, 10, 3390. [Google Scholar] [CrossRef]
- Shen, W.X.; Zang, J.H.; Hu, H.; Xu, J.M.; Zhang, Z.F.; Yan, R.Q.; Dai, S.G. Controlled synthesis of KCu7S4/rGO nanocomposites for electrochemical energy storage. Mater. Des. 2020, 195, 108992. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Zang, J.; Zhang, Z.; Wang, Y.; Lou, Q.; Bai, Y.; Fu, J.; Zhuang, C.; Zhang, Y.; et al. Robust VS4@rGO nanocomposite as a highcapacity and long-life cathode material for aqueous zinc-ion batteries. Nanoscale 2021, 13, 12370–12378. [Google Scholar] [CrossRef]
- Meng, H.Y.; Wang, S.L.; Ma, X.Y.; Zhang, D.H.; Zhang, L.L.; Liu, X.Y.; Zhang, L. Matching CP@NCOH/NF Cathode and GH/FNP/NF Anode for High-Performance Asymmetric Supercapacitor. Small 2023, 19, 2207496. [Google Scholar] [CrossRef]
- Wang, C.X.; Liu, Y.W.; Sun, Y.S.; Cui, L.; Liu, J.Q. 3D nanoflower-like and core–shell structured MCo2O4@MCo2S4@polypyrrole (M = Cu, Mn) composites as supercapacitor electrode materials with ultrahigh specific capacitances. J. Mater. Chem. A 2023, 11, 7639–7651. [Google Scholar] [CrossRef]
- Abbas, Z.; Hussain, N.; Ahmed, I.; Mobin, S.M. Cu-Metal Organic Framework Derived Multilevel Hierarchy (Cu/CuxO@NC) as a Bifunctional Electrode for High-Performance Supercapacitors and Oxygen Evolution Reaction. Inorg. Chem. 2023, 62, 8835–8845. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Tang, C.; Li, C.; Li, H.; Wang, Y.; Gong, H. The sol-gel-derived nickel-cobalt oxides with high supercapacitor performances. J. Electrochem. Soc. 2011, 158, A695–A699. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, S.C.; Qiao, G.Q.; Lu, G.X.; Cui, H.Z.; Wang, X.Z. Fabrication of Three-Dimensional Porous NiO/Amorphous Ni(OH)2 Composites for Supercapacitors. Energy Fuels 2020, 34, 16783–16790. [Google Scholar] [CrossRef]
- Xin, Y.F.; Dai, X.; Lv, G.J.; Wei, X.D.; Li, S.; Li, Z.Q.; Xue, T.; Shi, M.; Zou, K.Y.; Chen, Y.Z.; et al. Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors. ACS Appl. Mater. Interfaces 2021, 13, 28118–28128. [Google Scholar] [CrossRef] [PubMed]
- Bhagwan, J.; Han, J.I. Promotive Effect of MWCNTs on α-NiS Microstructure and Their Application in Aqueous Asymmetric Supercapacitor. Energy Fuels 2022, 36, 15210–15220. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liu, Y.X.; Wang, H.Q.; Liu, W.; Li, Y.; Zhang, J.F.; Hou, H.; Yang, J.L. Ultrathin NiCo-MOF Nanosheets for High-Performance Supercapacitor Electrodes. ACS Appl. Energy Mater. 2019, 2, 2063–2071. [Google Scholar] [CrossRef]
- Wang, Y.G.; Song, Y.F.; Xia, Y.Y. Electrochemical capacitors: Mechanism, materials. systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Cericola, D.; Kotz, R. Hybridization of rechargeable batteries and electrochemical capacitors: Principles and limits. Electrochim. Acta 2012, 782, 1–17. [Google Scholar] [CrossRef]
- Thounthong, P.; Raël, S.; Davat, B. Energy management of fuel cell/battery/supercapacitor hybrid power source for vehicle applications. J. Power Sources 2009, 193, 376–385. [Google Scholar] [CrossRef]
- Gogosti, Y. What nano can do for energy storage. ACS Nano 2014, 8, 5369–5371. [Google Scholar]
- Wang, K.B.; Chen, C.Y.; Li, Y.H.; Hong, Y.; Wu, H.; Zhang, C.; Zhang, Q.C. Insight into Electrochemical Performance of Nitrogen Doped Carbon/NiCo-Alloy Active Nanocomposites. Small 2023, 19, 2300054. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, J. Critical requirements for rapid charging of rechargeable Al-and Li-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 9452–9455. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Marinova, D.; Kalapsazova, M.; Zlatanova, Z.; Mereacre, L.; Zhecheva, E.; Stoyanova, R. Lithium Manganese Sulfates as a New Class of Supercapattery Materials at Elevated Temperatures. Materials 2023, 16, 4798. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhou, H.; Qin, B.; Zhao, N.; Lv, B.L. Ultrathin Nanosheet-Assembled, Phosphate Ion-Functionalized NiO Microspheres as Efficient Supercapacitor Materials. ACS Appl. Energy Mater. 2020, 3, 9980–9988. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.M.; Gao, L.J.; Zhang, D.; Guo, Y.Y.; Xu, R.Q. NiO Nanoparticles Anchored on N-Doped Laser-Induced Graphene for Flexible Planar Micro-Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 11314–11323. [Google Scholar] [CrossRef]
- Wang, G.S.; Yan, Z.X.; Wang, N.H.; Xiang, M.; Xu, Z.H. NiO/Ni Metal-Organic Framework Nanostructures for Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2021, 4, 9034–9043. [Google Scholar] [CrossRef]
- Meng, G.; Yang, Q.; Wu, X.C.; Wan, P.B.; Li, Y.P.; Lei, X.D.; Sun, X.M.; Liu, J.F. Hierarchical mesoporous NiO nanoarrays with ultrahigh capacitance for aqueous hybrid supercapacitor. Nano Energy 2016, 30, 831–839. [Google Scholar] [CrossRef]
- Gao, D.Y.; Liu, R.N.; Han, D.D.; Xu, P.C.; Wang, P.; Wei, Y. Constructing Ni-Co PBA derived 3D/1D/2D NiO/NiCo2O4/NiMn-LDH hierarchical heterostructures for ultrahigh rate capability in hybrid supercapacitors. J. Mater. Chem. A 2023, 11, 9546–9554. [Google Scholar] [CrossRef]
- Wu, X.L.; Zeng, F.; Song, X.Y.; Sha, X.F.; Zhou, H.T.; Zhang, X.G.; Liu, Z.; Yu, M.H.; Jiang, C.Z. In-situ growth of Ni(OH)2 nanoplates on highly oxidized graphene for all-solid-state flexible supercapacitors. Chem. Eng. J. 2023, 456, 140947. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, Y.H.; Chen, M.Y.; Liu, G.F.; Qi, P.C.; Wu, H.; Tang, Y.W. Multi-dimensional Ni(OH)2/(Ni(OH)2(NiOOH).167).857@Ni3S2 hierarchical structure for high-performance asymmetric supercapacitor. Appl. Surf. Sci. 2023, 6, 155625. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.L.; Zhang, X.Q.; Chen, D.Y.; Lin, Q.L. A novel Ni(OH)2/graphene nanosheets electrode with high capacitance and excellent cycling stability for pseudocapacitors. J. Power Sources 2016, 9, 153. [Google Scholar] [CrossRef]
- Han, C.; Si, H.Z.; Sang, S.B.; Liu, K.Y.; Liu, H.T.; Wu, Q.M. Carbon Dots Doped with Ni(OH)2 as Thin-Film Electrodes for Supercapacitors. ACS Appl. Nano Mater. 2020, 3, 12106–12114. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Xu, T.T.; Dai, S.G.; Yan, C.C.; Ma, C.Y.; Wang, X.C.; Xu, J.M.; Zhang, S.; Wang, Y. 3D mesoporous Ni(OH)2/WS2 nanofibers with highly enhanced performance for energy storage devices. Energy Technol. 2019, 7, 1800476. [Google Scholar] [CrossRef]
- Ren, X.C.; Guo, C.L.; Xu, L.Q.; Li, T.T.; Hou, L.F.; We, Y.H. Facile synthesis of hierarchical mesoporous honeycomb-like NiO for aqueous asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 19930–19940. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.F.; Wang, X.; Cui, M.Q.; Darmawan, P.; Wang, J.X.; Eh, A.L.; Lee, P.S. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Krishna, P.G.; Debendra, A.; Indu, B.; Vivek, S.; Maya, D.; Shova, N.; Jyotendra, K.; Kisan, C.; Amar, P.Y. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar]
- Hong, Y.W.; Yang, J.X.; Choi, W.M.; Wang, J.J.; Xu, J.L. B-Doped g-C3N4 Quantum Dots-Modified Ni(OH)2 Nanoflowers as an Efficient and Stable Electrode for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 1496–1504. [Google Scholar] [CrossRef]
- Jiang, W.C.; Yu, D.S.; Zhang, Q.; Goh, K.L.; Wei, L.; Yong, Y.L.; Jiang, R.G.; Wei, J.; Chen, Y. Ternary hybrids of amorphous nickel hydroxide-carbon nanotube-conducting polymer for supercapacitors with high energy density, excellent rate capability, and long cycle life. Adv. Funct. Mater. 2015, 25, 1063–1073. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhang, Q.B.; Chen, D.C.; Cheng, Y.; Deng, X.; Chen, Y.; Murphy, R.; Xiong, X.H.; Song, B.; et al. Rational Design of Nickel Hydroxide-Based Nanocrystals on Graphene for Ultrafast Energy Storage. Adv. Energy Mater. 2017, 8, 1702247. [Google Scholar] [CrossRef]
- Wan, N.W.K.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Foo, C.Y.; Jiang, Z.T. Electrochemical performance of aqueous hybrid supercapacitor based on LiFePO4/Si/graphene composite. Chem. Eng. J. 2023, 456, 141132. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ma, Q.; Zhang, H.B.; Zhang, Q.H.; Zhang, K.L.; Mei, H.; Xu, B.; Sun, D.F. Self-Assembly of Metal-Organic Frameworks on Graphene Oxide Nanosheets and In Situ Conversion into a Nickel Hydroxide/Graphene Oxide Battery-Type Electrode. Inorg. Chem. 2022, 61, 12129–12137. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Gao, Y.H.; Li, Y.Y.; Sun, X.; Chen, S.L.; Li, M.C. Ni(OH)2 Nanosheets Grown on Reduced Graphene Oxide for Supercapacitor Electrodes. ACS Appl. Nano Mater. 2022, 5, 7471–7480. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Rad, H.F. In situ growth of Ni(OH)2-porous nitrogen-doped graphene composite onto Ni foam support as advanced electrochemical supercapacitors materials. J. Mater. Sci. Mater. Electron. 2022, 33, 11038–11054. [Google Scholar] [CrossRef]
- Girish, S.G.; Deepak, P.D.; Sujata, S.S.; Chandrakant, D.L. One step hydrothermal synthesis of micro-belts like β-Ni(OH)2 thin films for supercapacitors. Ceram. Int. 2013, 1, 91. [Google Scholar]
- Ji, J.Y.; Zhang, L.L.; Ji, H.X.; Li, Y.; Zhao, X.; Bai, X.; Fan, X.B.; Zhang, F.B.; Ruoff, R.S. Nanoporous Ni(OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor. ACS Nano 2013, 7, 6237–6243. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Deng, D. Bio-inspired synthesis of α-Ni(OH)2 nanobristles on various substrates and their applications. J. Mater. Chem. A 2016, 4, 6919–6925. [Google Scholar] [CrossRef]
- Du, H.M.; Wang, Y.J.; Yuan, H.T.; Jiao, L.F. Facile synthesis and high capacitive performance of 3D hierarchical Ni(OH)2 microspheres. Electrochim. Acta 2016, 196, 84–91. [Google Scholar] [CrossRef]
- Ambade, R.B.; Lee, H.; Lee, K.H.; Lee, H.; Veerasubramani, G.K.; Kim, Y.B.; Han, T.H. Ultrafast flashlight sintered mesoporous NiO nanosheets for stable asymmetric supercapacitors. Chem. Eng. J. 2022, 135, 41. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Wang, S.; Zhou, S.J.; Xu, H.B.; Zhao, J.P.; Wang, J.; Li, Y. An electrochromic supercapacitor based on an MOF derived hierarchical-porous NiO film. Nanoscale 2020, 12, 8934–8941. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Jiao, L.; Cao, K.; Wang, Y.; Yuan, H. Polyol-mediated synthesis of mesoporous alpha-Ni(OH)2 with enhanced supercapacitance. ACS Appl. Mater. Interfaces 2013, 5, 6643–6648. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Song, J.; Zhu, Y.; Cao, C. High-performance supercapacitor electrode based on amorphous mesoporous Ni(OH)2 nanoboxes. J. Power Sources 2014, 262, 344–348. [Google Scholar] [CrossRef]
- Li, Z.; Han, J.; Fan, L.; Wang, M.; Tao, S.; Guo, R. The anion exchange strategy towards mesoporous alpha-Ni(OH)2 nanowires with multinanocavities for high-performance supercapacitors. Chem. Commun. 2015, 51, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Duan, S.Y.; Shen, Y.; Fang, K.; Wang, Y.Z.; Lin, M.; Guo, X.F. In-situ-grown Mg(OH)2-derived hybrid α-Ni(OH)2 for highly stable supercapacitor. ACS Energy Lett. 2016, 1, 814–819. [Google Scholar] [CrossRef]
- Sun, W.P.; Rui, X.H.; Ulaganathan, M.; Madhavi, S.; Yan, Q.Y. Few-layered Ni(OH)2 nanosheets for high-performance supercapacitors. J. Power Sources 2015, 295, 323–328. [Google Scholar] [CrossRef]
- Alhebshi, N.A.; Rakhi, R.B.; Alshareef, H.N. Conformal coating of Ni(OH)2 nanoflakes on carbon fibers by chemical bath deposition for efficient supercapacitor electrodes. J. Mater. Chem. A 2013, 1, 14897–14903. [Google Scholar] [CrossRef]
- Li, L.; Tan, L.; Li, G.N.; Zhang, Y.M.; Liu, L.L. Self-templated synthesis of porous Ni(OH)2 nanocube and its high electrochemical performance for supercapacitor. Langmuir 2017, 33, 12087–12094. [Google Scholar] [CrossRef]
- Kore, R.M.; Lokhande, B.J. Hierarchical mesoporous network of amorphous a-Ni(OH)2 for high performance supercapacitor electrode material synthesized from a novel solvent deficient approach. Electrochim. Acta 2017, 245, 780–790. [Google Scholar] [CrossRef]
- Aguilera, L.; Leyet, Y.; Garcia, R.P.; Hernández, E.P.; Passos, R.R.; Pocrifka, L.A. Cabbage-like α-Ni(OH)2 with a good long-term cycling stability and high electrochemical performances for supercapacitor applications. Chem. Phys. Lett. 2017, 677, 75–79. [Google Scholar] [CrossRef]
- Xiong, X.H.; Ding, D.; Chen, D.C.; Waller, G.; Bu, Y.F.; Wang, Z.X.; Liu, M.L. Three-dimensional ultrathin Ni(OH)2 nanosheets grown on nickel foam for high-performance supercapacitors. Nano Energy 2015, 11, 154–161. [Google Scholar] [CrossRef]
- Li, L.J.; Xu, J.; Lei, J.L.; Zhang, J.; McLarnon, F.; Wei, Z.D.; Li, N.B.; Pan, F.S. A one-step, cost-effective green method to in situ fabricate Ni(OH)2 hexagonal platelets on Ni foam as binder-free supercapacitor electrode materials. J. Mater. Chem. A 2015, 3, 1953–1960. [Google Scholar] [CrossRef]
- Cui, H.T.; Xue, J.Y.; Ren, W.Z.; Wang, M.M. Ultra-high specific capacitance of β-Ni(OH)2 monolayer nanosheets synthesized by an exfoliation-free sol-gel route. J. Nanopart. Res. 2014, 16, 2601. [Google Scholar] [CrossRef]
- Naveen, N.; Park, C.; Sohn, K.S.; Pyo, M. Nickel hydroxide nanoplatelets via dendrimer-assisted growth on graphene for high-performance energy-storage applications. Electrochim. Acta 2017, 248, 313–321. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Na, H.; Pan, C.; Xu, X.; Huang, G.; Liu, Y.; Liu, Y.; Gao, J. Self-assembly method to fabricate reduced graphene oxide aerogels loaded with nickel hydroxyl nanoparticles and their excellent properties in absorbing and supercapacitors. Ind. Eng. Chem. Res. 2016, 55, 6553–6562. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zhang, D.; Sun, X.; Lin, H.; Wang, C.; Ma, Y. One-step electrophoretic deposition of reduced graphene oxide and Ni(OH)2 composite films for controlled syntheses supercapacitor electrodes. J. Phys. Chem. B 2013, 117, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lu, X. A solid-state reaction route to anchoring Ni(OH)2 nanoparticles on reduced graphene oxide sheets for supercapacitors. Ind. Eng. Chem. Res. 2012, 51, 9973–9979. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632–2841. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Sun, C.; Feng, Z.; Yang, B. Controlled synthesis of Ni(OH)2/graphene composites and their transformation to NiO/graphene for energy storage. Electrochim. Acta 2016, 212, 390–398. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, S. Nanocomposites of Ni(OH)2/reduced graphene oxides with controllable composition, size, and morphology: Performance variations as pseudocapacitor electrodes. ChemPlusChem 2012, 77, 807–816. [Google Scholar] [CrossRef]
- Min, S.; Zhao, C.; Zhang, Z.; Chen, G.; Qian, X.; Guo, Z. Synthesis of Ni(OH)2/RGO pseudocomposite on nickel foam for supercapacitors with superior performance. J. Mater. Chem. A 2015, 3, 3641–3650. [Google Scholar] [CrossRef]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 131, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, W.; Wei, T.; Zhang, Q.; Fan, Z.J.; Wei, F. Fabrication and electrochemical performances of hierarchical porous Ni(OH)2 nanoflakes anchored on graphene sheets. J. Mater. Chem. 2012, 22, 11494–11502. [Google Scholar] [CrossRef]
- Lee, J.W.; Ahn, T.; Soundararajan, D.; Koc, J.M.; Kim, J.D. Non-aqueous approach to the preparation of reduced graphene oxide/α-Ni(OH)2 hybrid composites and their high capacitance behavior. Chem. Commun. 2011, 22, 6305–6307. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.L.; Chen, Z.D.; Liu, X.; Wu, Z.F.; Zheng, C.H. Homogeneous growth of nano-sized β-Ni(OH)2 on reduced graphene oxide for high-performance supercapacitors. Electrochim. Acta 2012, 81, 321–329. [Google Scholar] [CrossRef]
- Wang, R.H.; Jayakumar, A.; Xu, C.; Lee, J.M. Ni(OH)2 nanoflowers/graphene hydrogels: A new assembly for supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 3736–3742. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, S.G.; Fouadc, H.; Cho, M.H. Intercalated reduced graphene oxide and its content effect on the supercapacitance performance of the three dimensional flower-like β-Ni(OH)2 architecture. New J. Chem. 2017, 41, 10467–10475. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Yan, X. Synergistic effect between ultra-small nickel hydroxide nanoparticles and reduced graphene oxide sheets for the application in highperformance asymmetric supercapacitor. Sci. Rep. 2015, 5, 11095. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, J.; Tan, L.; Qi, S.P.; Li, B.L.; Chen, J.X.; Li, J.; Lou, Y.B.; Guo, Y.Z. Morphological and electronic modification of NiS2 for efficient supercapacitors and hydrogen evolution reaction. J. Power Sources 2023, 577, 233239. [Google Scholar] [CrossRef]
- Liu, J.P.; Pu, Y.; Zhang, Q.Y.; Cheng, Z.Y.; Zheng, Y.; Wang, Y.C.; Liu, W.; Li, S.Y.; Zhang, J.X. Tracking surface ionic movement of Ni3S2@CuS electrode materials with high electrochemical performance. Chem. Eng. J. 2023, 461, 141910. [Google Scholar] [CrossRef]
- Shoeb, M.; Mashkoor, F.; Abdul, H.J.; Anwer, H.; Zhu, S.S.; Zahid, A.M.; Changyoon, J. VARTM-assisted high-performance solid-state structural supercapacitor device based on the synergistic effect of Ni(OH)2-Co3S4 nanocomposite for widened potential window and charge storage mechanism. Chem. Eng. J. 2023, 466, 143116. [Google Scholar] [CrossRef]
- Azimov, F.; Lee, J.; Park, S.; Hyun, M.J. Fabrication of Assembled FeS2 Nanosheet and Application for HighPerformance Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2023, 15, 26967–26976. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Telpande, S.; Mahapatra, A.D.; Kumarb, P.; Misra, A. Role of the electrode-edge in optically sensitive three-dimensional carbon foam-MoS2 based highperformance micro-supercapacitors. J. Mater. Chem. A 2023, 11, 4963–4976. [Google Scholar] [CrossRef]
- Hu, J.J.; Shi, Y.; Sun, L.; Xie, F.; Gao, K.Y.; Qu, Y.R.; Tan, H.K.; Zhang, Y.H. MOF-derived spherical NixSy/carbon with B-doping enabling high supercapacitive performance. J. Mater. Sci. Technol. 2013, 153, 219–227. [Google Scholar] [CrossRef]
- Kushwaha, V.; Gupta, A.; Choudhary, R.B.; Mandal, K.D.; Mondalc, R.; Singh, P. Nanocrystalline β-NiS: A redox-mediated electrode in aqueous electrolyte for pseudocapacitor/supercapacitor applications. Phys. Chem. Chem. Phys. 2023, 25, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, K.; Deng, Y. The synthesis of nickel sulfide deposited with nitrogen-doped carbon quantum dots as advanced electrode materials for supercapacitors. J. Mater. Sci. 2022, 57, 14052–14064. [Google Scholar] [CrossRef]
- Shi, Y.; Qu, Y.R.; Tan, H.K.; Sun, L.; Sun, C.; Fan, K.F.; Hu, J.J.; Wang, K.; Zhang, Y.H. RGO-loaded double phase Mo-doped NiS for enhanced battery-type energy storage in hybrid supercapacitors. Electrochim. Acta 2022, 426, 140810. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Gao, S.Y.; Zhang, J.W.; Wang, J.R.; She, W.N.; Wang, K.J.; Xia, X.; Yang, B.; Meng, X.X. Facile solid-phase synthesis of layered NiS/rGO nanocomposite for high-performance hybrid supercapacitor. J. Power Sources 2021, 514, 230590. [Google Scholar] [CrossRef]
- Que, T.N.; Umesh, T.N.; Chen, J.Y.; Soojin, P.; Sungjune, P. Ceria nanoflowers decorated Co3O4 nanosheets electrodes for highly efficient electrochemical supercapacitors. Appl. Surf. Sci. 2023, 613, 156034. [Google Scholar]
- He, Y.; Zhou, W.Q.; Li, D.Q.; Liang, Y.M.; Chao, S.X.; Zhao, X.Q.; Zhang, M.M.; Xu, J.K. Rare Earth Doping Engineering Tailoring Advanced Oxygen-Vacancy Co3O4 with Tunable Structures for High-Efficiency Energy Storage. Small 2023, 19, 2206956. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.J.; Shi, M.P.; Ma, L.N.; Huang, Y.D. Hierarchical polygon Co3O4 flakes/N, O-dual doped porous carbon frameworks for flexible hybrid supercapacitors. Electrochim. Acta 2022, 424, 140631. [Google Scholar] [CrossRef]
- Yang, C.Q.; Li, W.Q.; Liu, X.Q.; Song, X.M.; Li, H.P.; Tan, L.C. Preparation of MoFs-Derived Cobalt Oxide/Carbon Nanotubes Composites for High-Performance Asymmetric Supercapacitor. Molecules 2023, 28, 3177. [Google Scholar] [CrossRef]
- Merum, D.; Pitcheri, R.; Gonuguntla, V.; Joo, S.W.B. Synthesis and properties of oxygen-deficient cobalt oxide nanocubes for supercapacitor application. Mater. Lett. 2023, 347, 134585. [Google Scholar] [CrossRef]
- Liu, Q.P.; Zhou, Q.P.; Gao, C.S.; Liu, L.; Ye, H.Y. Excellent electrochemical stability of Co3O4 array with carbon hybridization derived from metal-organic framework. Nanotechnology 2021, 32, 485710. [Google Scholar] [CrossRef]
- Sivakumar, P.; Raj, C.; Justin, J.H.; Park, H.S. 2D/2D nanoarchitecture of Ni/NiCo2O4 deposited onto reduced graphene oxide for high-performance hybrid supercapacitor applications. J. Energy Storage 2023, 69, 107946. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Pourhosseini, S.E.M.; Norouzi, O.; Naderi, H.R. Designing 3D ternary hybrid composites composed of graphene, biochar and manganese dioxide as high-performance supercapacitor electrodes. Nanomaterials 2023, 13, 1866. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.K.; Ghosh, T.; Kumar, R. Improved inclusive performance of bi-stacked NiO nanoflakes coated nano-Co3O4 for dual function: An electrochromic-supercapacitor. J. Energy Storage 2023, 67, 107643. [Google Scholar] [CrossRef]
- Silambarasan, S.; Sivakumar, M.; Kumar, K.R.; Jiang, Z.Q.; Maiyalagan, T. Nickel-doped Co3O4 spinel nanospheres embedded in nitrogen-doped carbon composites derived from bimetallic NiCo metal-organic framework as a high-performance asymmetric supercapacitor. New J. Chem. 2023, 47, 8649–8660. [Google Scholar] [CrossRef]
- Yang, D.D.; Xu, M.; Liang, X.; Wang, J.Y.; Fang, W.Y.; Zhu, C.G.; Wang, F.W. Facile synthesis of Pr-doped Co3O4 nanoflakes on the nickel-foam for high performance supercapacitors. Electrochim. Acta 2022, 406, 139815. [Google Scholar] [CrossRef]
- Zhang, H.; Geng, S.Y.; Ouyang, M.Z.; Mao, M.X.; Xie, F.; Riley, D.J. Using Metal Cation to Control the Microstructure of Cobalt Oxide in Energy Conversion and Storage Applications. Small 2022, 18, 2106391. [Google Scholar] [CrossRef]
- Cong, Y.; Jiang, T.T.; Dai, Y.M.; Wu, X.F.; Lv, M.Y.; Chen, M.T.; Ye, T.W.; Wu, Q. 3D network structure of Co(OH)2 nanoflakes/Ag dendrites via a one-step electrodeposition for high-performance supercapacitors. Mater. Res. Bull. 2023, 157, 112013. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Qiu, Y.L.; Zhang, A.T.; Yang, W.R.; Barrow, C.J.; Razal, J.M.; Li, A.H.; Liu, J.Q. In-situ formation of α-Co(OH)2 nanosheet arrays on magnesium cobaltate nanowires for hybrid supercapacitors with enhanced electrochemical performance. Appl. Surf. Sci. 2021, 568, 150856. [Google Scholar] [CrossRef]
- Hao, J.Y.; Yan, J.; Hu, Q.; Bai, Y.C.; Zhou, Y.; Zou, X.F.; Xiang, B. Design of Co(OH)2 composite electrode with high active surface area by sulfur control and graphene encapsulation strategies. Appl. Surf. Sci. 2022, 596, 153612. [Google Scholar] [CrossRef]
- Wang, X.X.; Liu, K.H.; Li, J.; Liu, Y.Y.; Wang, M.R.; Cui, H.T. Creation of an extrinsic pseudocapacitive material presenting extraordinary cycling-life with the battery-type material Co(OH)2 by S2− doping for application in supercapacitors. Chem. Eng. J. 2023, 451, 138969. [Google Scholar] [CrossRef]
- Zheng, L.X.; Xu, P.H.; Zhao, Y.J.; Peng, J.X.; Yang, P.J.; Shi, X.W.; Zheng, H.J. Unique core-shell Co2(OH)2CO3@MOF nanoarrays with remarkably improved cycling life for high performance pseudocapacitors. Electrochim. Acta 2022, 412, 140142. [Google Scholar] [CrossRef]
- Li, J.B.; Li, Z.H.; Zhan, F.; Shao, M.F. Phase engineering of cobalt hydroxide toward cation intercalation. Chem. Sci. 2021, 12, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, L.; Shen, J.; Li, Z.H.; Liu, S.Y. Metal-organic frameworks induced robust layered Co(OH)2 nanostructures for ultra-high stability hybrid supercapacitor electrodes in aqueous electrolyte. J. Power Sources 2020, 477, 228974. [Google Scholar]
- Gong, W.Z.; Wang, M.J.; An, Y.; Wang, J.L.; Zhou, L.X.; Xia, Y.; Wang, C.J.; Dong, K.; Pan, C.; Zhou, R.F. Rational design and synthesis of hydrotalcite-like α-Co(OH)2 nanoflakes for extrinsic pseudocapacitive electrodes with superb cycling stability. J. Energy Storage 2021, 38, 102579. [Google Scholar] [CrossRef]
- Gan, Z.W.; Ren, X.H.; Sun, Y.X.; Sun, M.X.; Yan, Y.J.; Cao, B.B.; Shen, W.Z.; Yu, H.J.; Li, Z.J.; Fu, Y.Q. Highly porous nanocomposites of Mn doped cobalt-based hydroxide/sulfide as high-performance electrode materials for hybrid supercapacitors. J. Energy Storage 2023, 69, 107934. [Google Scholar] [CrossRef]
- Wang, E.T.; Jiang, S.S.; Bu, X.D. One-pot electrochemical assembling of porous cobalt hydroxide/nitrogen-doped porous graphene onto Ni foam as a binder-free electrode for supercapacitor applications. J. Energy Storage 2020, 32, 101881. [Google Scholar] [CrossRef]
- Salunkhe, A.D.; Pawar, P.S.; Torane, A.P. MOF derived NiCo2O4 nanosheets for high performance asymmetric supercapacitor. J. Electroanal. Chem. 2023, 939, 117475. [Google Scholar] [CrossRef]
- Ghaemi, S.P.; Masoudpanah, S.M.; Alamolhoda, S. CTAB-assisted synthesis of porous cuboid NiCo2O4 powders for high-performance supercapacitor. J. Energy Storage 2023, 67, 107635. [Google Scholar] [CrossRef]
- Pore, O.C.; Fulari, A.V.; Lohar, G.M. Synthesis of NiCo2O4 microflowers by facile hydrothermal method: Effect of precursor concentration. Chem. Phys. Lett. 2023, 824, 140551. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Li, H.; Tang, R.Y.; Wang, X.Y.; Wu, Y.; Shi, Y.; Zhang, Y. Photo-assisted asymmetric supercapacitors based on dual photoelectrodes for enhanced photoelectric energy storage. J. Mater. Chem. A 2023, 11, 15844–15854. [Google Scholar] [CrossRef]
- Yang, W.D.; Xiang, J.; Wu, F.F. Nanoengineering of ZnCo2O4@CoMoO4 heterogeneous structures for supercapacitor and water splitting applications. Ceram. Int. 2022, 49, 4422–4434. [Google Scholar] [CrossRef]
- Ma, Q.H.; Cui, F.; Cui, T.Y. Built-in electric field boosted ionic transport kinetics in the heterostructured ZnCo2O4/ZnO nanobelts for high-performance supercapacitor. J. Colloid Interface Sci. 2023, 629, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Siyal, S.H.; Bocchetta, P. Flower-like Highly Open-Structured Binder-Free Zn-Co-Oxide Nanosheet for High-Performance Supercapacitor Electrodes. Molecules 2022, 27, 4850. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, I. Hybrid nanostructures made of porous binary transition metal oxides for high performance asymmetric supercapacitor application. J. Energy Storage 2023, 67, 107530. [Google Scholar]
- Karthik, R.; Sukanya, R.; Chen, S.M.; Hasan, M.; Dhakal, G.; Shafi, P.M.; Shim, J.J. Development of an Amorphous Nickel Boride/Manganese Molybdate Heterostructure as an Efficient Electrode Material for a High-Performance Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2023, 15, 11927–11939. [Google Scholar] [CrossRef]
- Li, P.X.; Qiao, D.W.; Bai, J.K.; Li, X.L. Facile synthesis of petal-like MnMoO4/Ni(OH)2 ultrathin nanosheets for high-performance asymmetric supercapacitor. Mater. Lett. 2023, 337, 134007. [Google Scholar] [CrossRef]
- Nandagopal, T.; Balaji, G.; Vadivel, S. Enhanced electrochemical performance of CoMoO4 nanorods/reduced graphene oxide (rGO) as asymmetric supercapacitor devices. J. Energy Storage 2023, 68, 107710. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Huang, X.; Yin, H.Q.; Zhang, K.Y.; Qin, A.M.; Chen, S.P. In-situ growth of KCu7S4@CoMoO4 core-shell structure on Ni foam for high performance supercapacitor electrode. J. Alloys Compd. 2022, 927, 166996. [Google Scholar] [CrossRef]
- Liu, L.Y.; Dai, D.F.; Yang, B.; Li, B.; Liu, X.Y. Green preparation of CoMoO4 nanoparticles through a mechanochemical method for energy storage applications. New J. Chem. 2022, 46, 23369–23378. [Google Scholar] [CrossRef]
- Zhou, J.H.; Chen, Z.X.; Yang, H.; Liu, M.J.; Gu, B.N.; Chen, C.C.; Lv, H.F.; Zhou, Z.Y.; Chueh, Y.L. Hierarchically Hybrid Porous Co3O4@NiMoO4/CoMoO4 Heterostructures for High-Performance Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 8282–8296. [Google Scholar]
- Shen, L.F.; Yu, L.; Yu, X.Y.; Zhang, X.G.; Lou, X.W. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.C.; Liu, F.Z.; Xua, L.; Yang, S.C. Urchin-like NiCo2O4 hollow microspheres and FeSe2 micro-snowflakes for flexible solid-state asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 5568–5576. [Google Scholar] [CrossRef]
- Li, Q.; Lu, C.X.; Chen, C.M.; Xie, L.J.; Liu, Y.D.; Li, Y.; Kong, Q.Q.; Wang, H. Layered NiCo2O4/reduced graphene oxide composite as an advanced electrode for supercapacitor. Energy Storage Mater. 2017, 8, 59–67. [Google Scholar] [CrossRef]
- Al-Rubaye, S.; Rajagopalan, R.; Dou, S.X.; Cheng, Z.X. Facile synthesis of a reduced graphene oxide wrapped porous NiCo2O4 composite with superior performance as an electrode material for supercapacitors. J. Mater. Chem. A 2017, 5, 18989–18997. [Google Scholar] [CrossRef]
- Sun, S.M.; Wang, S.; Li, S.D.; Li, Y.N.; Zhang, Y.H.; Chen, J.L.; Zhang, Z.H.; Fang, S.M.; Wang, P.Y. Asymmetric supercapacitors based on a NiCo2O4/three dimensional graphene composite and three dimensional graphene with high energy density. J. Mater. Chem. A 2016, 4, 18646–18653. [Google Scholar] [CrossRef]
- Ye, Y.; Luo, Y.Z.; Lou, J.T.; Chen, X.L.; Cheng, Y.J.; Xia, J.F.; Li, Y.B.; Guo, K.K. Hollow Spherical NiCo2S4@N-CNT Composites with High Energy Density for All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 6742–6751. [Google Scholar] [CrossRef]
- Abuali, M.; Arsalani, N.; Ahadzadeh, I. On the effect of polypyrrole on electrochemical performance of micro-sized hollow spheres of NiCo2S4 and CuCo2S4 nanoparticles. Electrochim. Acta 2022, 427, 140840. [Google Scholar] [CrossRef]
- Qi, S.H.; Wu, D.X.; Dong, Y.; Liao, J.Q.; Foster, C.W.; Feng, Y.Z.; Liu, C.T.; Ma, J.M. T Cobalt-based electrode materials for sodium-ion batteries. Chem. Eng. 2019, 3, 166. [Google Scholar] [CrossRef]
- Zhang, H.B.; Lv, Y.; Wu, X.Y.; Guo, J.X.; Jia, D.Z. Electrodeposition synthesis of reduced graphdiyne oxide/NiCo2S4 hierarchical nanosheet arrays for small size and light weight aqueous asymmetry supercapacitors. J. Alloys Compd. 2023, 947, 169403. [Google Scholar] [CrossRef]
- Peng, T.Q.; Yi, H.; Sun, P.; Jing, Y.T.; Wang, R.J.; Wang, H.W.; Wang, X.F. In situ growth of binder-free CNTs@Ni-Co-S nanosheets core/shell hybrids on Ni mesh for high energy density asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 8888–8897. [Google Scholar] [CrossRef]
- Yuan, K.; Gao, T.J.; Yang, Y.; Luo, W.; Li, S.; Zhang, C.Y.; Xu, J.X.; Li, N.; Zhu, Y.R. Template sacrificial controlled synthesis of hierarchical nanoporous carbon@NiCo2S4 microspheres for high-performance hybrid supercapacitors. Rare Met. 2023, 42, 2643–2657. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ma, M.Z.; Yang, J.; Sun, C.C.; Su, H.Q.; Huang, W.; Dong, X.C. Shape-controlled synthesis of NiCo2S4 and their charge storage characteristics in supercapacitors. Nanoscale 2014, 6, 9824–9830. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.J.; Dutta, J.C.; Sahu, P.P. Confined growth of NiCo2S4 on 2D/2D porous carbon self-repairing g-C3N4/rGO heterostructure for enhanced performance of asymmetric supercapacitors. Chem. Eng. J. 2023, 463, 142376. [Google Scholar] [CrossRef]
- Dai, S.G.; Xi, Y.; Hu, C.G.; Yue, X.L.; Cheng, L.; Wang, G. MnO2@KCu7S4 NWs hybrid compositions for high-power all-solid-state supercapacitor. J. Power Sources 2015, 274, 477–482. [Google Scholar] [CrossRef]
- Dai, S.G.; Xi, Y.; Hu, C.G.; Yue, X.L.; Cheng, L.; Wang, G. Different proportions of C/KCu7S4 hybrid structure for high-performance supercapacitors. J. Power Sources 2014, 263, 175–180. [Google Scholar] [CrossRef]
- Guo, X.L.; Zhang, J.M.; Xu, W.N.; Hu, C.G.; Sun, L.D.; Zhang, Y.X. Growth of Ni/Mn LDH nanosheet arrays on KCu7S4 microwires for hybrid supercapacitors with enhanced electrochemical performance. J. Mater. Chem. A 2017, 5, 20579–20587. [Google Scholar] [CrossRef]
- Dai, S.G.; Xi, Y.; Hu, C.G.; Hu, B.S.; Yue, X.L.; Cheng, L.; Wang, G. C@KCu7S4 microstructure for solid-state supercapacitors. RSC Adv. 2014, 4, 40542–40545. [Google Scholar] [CrossRef]
- Tang, J.H.; Ge, Y.C.; Shen, J.F.; Ye, M.X. Facile synthesis of CuCo2S4 as a novel electrode material for ultrahigh supercapacitor performance. Chem. Commun. 2016, 52, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Kenesi, A.G.; Ghorbani, M.; Lashkenari, M.S. High electrochemical performance of PANI/CdO nanocomposite based on graphene oxide as a hybrid electrode materials for supercapacitor application. Int. J. Hydrog. Energy 2019, 47, 38849–38861. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Yadav, H.M.; Lee, Y.J.; Sivasamy, A.; Kathalingam, A.; Kim, H.S.; Kim, J.H.; Kim, H.S. Fabrication of CuCo2S4 on composite interface materials made of polypyrrole and nitrogen-doped carbon nanotubes for use in supercapacitors. J. Energy Storage 2023, 67, 107518. [Google Scholar] [CrossRef]
- Kuo, T.R.; Lin, K.H.; Chen, M.W.; Yougbaré, S.; Lin, L.Y.; Wu, Y.F. Tailing copper cobalt sulfide particle-decorated tube-like structure as efficient active material of battery supercapacitor hybrid. J. Energy Storage 2023, 67, 107564. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.W.; Liu, L.; Fautrelle, Y.; Lu, X.G.; Li, X. High Magnetic Field-Engineered Bunched Zn–Co–S Yolk–Shell Balls Intercalated within S, N Codoped CNT/Graphene Films for Free-Standing Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 33690–33701. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ge, P.B.; Shao, Y.Q.; Liu, Z.J.; Li, H.H.; Chen, K.F.; Xu, L.H.; Zhuang, J.H.; Chen, Y.X.; Xia, X.J. Zn-Co-S coatings with a rough and porous nano-dendrite structure for high-performance asymmetric supercapacitors without binder. Electrochim. Acta 2022, 429, 141048. [Google Scholar] [CrossRef]
- Manas, M.; Krishna, C.; Sarbaranjan, P.; Adwaita, K.; Malay, C.; Souvik, M.; Wonjae, S.; Sachindranath, D.; Changwoon, N.; Kumar, B.S. Electrodeposited Binder-free Mn-Co-S Nanosheets toward High Specific-Energy Aqueous Asymmetric Supercapacitors. ACS Appl. Electron. Mater. 2022, 4, 4357–4367. [Google Scholar]
- Haider, S.S.; Dad, S.; Zakar, S.; Iqbal, M.Z. Mechanistic scrutinizing the charge storage phenomena of battery-grade Mn-Co-S electrodes. Mater. Res. Bull. 2022, 156, 111953. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, C.L.; Fang, S.W.; Zhu, L.P.; Xu, F.; Sun, L.X.; Zou, Y.J.; Zhang, J. Template strategy to synthesize porous Mn-Co-S nanospheres electrode for high-performance supercapacitors. J. Energy Storage 2021, 44, 103267. [Google Scholar] [CrossRef]
- Ramtin, A.; Mohammadi, Z.A.; Davarani, S.S.H. Rational Construction of Core-Shell Ni−Mn−Co−S@Co(OH)2 Nanoarrays toward High-Performance Hybrid Supercapacitors. ChemElectroChem 2020, 7, 2816–2825. [Google Scholar]
- Moosakhani, S.; Ali, A.S.A.; Mohammadpour, R.; Ge, Y.L.; Hannula, S.P. Solution synthesis of CuSbS2 nanocrystals: A new approach to control shape and size. J. Alloys Compd. 2018, 736, 190–201. [Google Scholar] [CrossRef]
- Das, A.; Mohapatra, M.; Basu, S. Expeditious synthesis of 3D pyramidal faceted CuSbS2 architectures manifesting unrivalled pseudo capacitive energy storage. Mater. Lett. 2021, 289, 129412. [Google Scholar] [CrossRef]
- Javadian, S.; Bayat, E.; Parviz, Z.; Dalir, N.; Gharibi, H. New rationally designed hybrid polypyrrole@SnCoS4 as an efficient anode for lithium-ion batteries. New J. Chem. 2021, 45, 11737–11751. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jin, Y.H.; Song, Y.Y.; Wang, H.; Jia, M.Q. Induced bimetallic sulfide growth with reduced graphene oxide for high-performance sodium storage. J. Colloid Interface Sci. 2023, 642, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.L.; Qi, S.Y.; Li, Y.; Sun, T.; Liu, Y.G.; Yi, T.F. Towards high-performance anodes: Design and construction of cobalt-based sulfide materials for sodium-ion batteries. J. Energy Chem. 2021, 54, 680–698. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Liu, X.; Wang, W.; Peng, T.; Guo, P.; Sun, H.; Yan, H.; Luo, Y. NiCo2S4/carbon nanotube nanocomposites with a chain-like architecture for enhanced supercapacitor performance. CrystEngComm 2016, 18, 7696–7706. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Wu, H.B.; Lou, W. Formation of NixCo3−xS4 hollow nanoprisms with enhanced pseudocapacitive properties. Angew. Chem. 2014, 126, 3785–3788. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Qing, C.; Sun, D.M.; Wang, B.X.; Qu, G.; Sun, M.; Tang, Y.W. Construction of carbon-nickel cobalt sulphide hetero-structured arrays on nickel foam for high performance asymmetric supercapacitors. Electrochim. Acta 2015, 174, 1104–1112. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Wang, X.; Song, S.Y.; Lou, X.W. Formation of onion-like NiCo2S4 particles via sequential ion-exchange for hybrid supercapacitors. Adv. Mater. 2017, 29, 1605051. [Google Scholar] [CrossRef]
- Khumujam, D.D.; Kshetri, T.; Singh, T.I.; Kim, N.H.; Lee, J.H. Hierarchical Integrated Hybrid Structural Electrodes Based on Co-N/C and Mo-doped NiCo-LDH@Co-N/C Anchored on MX/CF for High Energy Density Fiber-Shaped Supercapacitor. Adv. Funct. Mater. 2023, 2302388. [Google Scholar] [CrossRef]
- Hu, H.X.; Li, K.D.; Li, X.S.; Wang, L.Y.; Yang, X.J.; Zhang, Q.X. Facile fabrication of NiCo-LDH on activated rice husk carbon for high-performance all-solid-state asymmetric supercapacitors. New J. Chem. 2023, 47, 14030–14038. [Google Scholar] [CrossRef]
- Nagaraju, G.; Raju, G.S.; Ko, Y.H.; Yu, J.S. Hierarchical Ni-Co layered double hydroxide nanosheets entrapped on conductive textile fibers: A cost-effective and flexible electrode for high-performance pseudocapacitors. Nanoscale 2016, 8, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Yam, K.M.; Zhang, C.; Fan, H.J.; Ho, G.W. In situ transformation of MOFs into layered double hydroxide embedded metal sulfdes for improved electrocatalytic and supercapacitive performance. Adv. Mater. 2017, 29, 1606814. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, Q.; Lu, Z.T.; Liu, J.Y.; Chen, R.R.; Li, R.M.; Song, D.L.; Jing, X.Y.; Liu, P.L.; Wang, J. Rational design of sandwiched Ni-Co layered double hydroxides hollow nanocages/graphene derived from metal-organic framework for sustainable energy storage. ACS Sustain. Chem. Eng. 2017, 5, 9923–9934. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.Q.; Zhang, G.G.; Xu, M.; Lu, W.; Zhou, L.M.; Huang, H.T. Design of hierarchical Ni-Co@Ni-Co layered double hydroxide core-shell structured nanotube array for high-performance flexible all-solid-state battery-type supercapacitors. Adv. Funct. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.L.; Yan, X.B.; Lu, M.Q.; Wang, L.Z.; Bell, J.; Wang, H.X. 2-Methylimidazole-derived Ni-Co layered double hydroxide nanosheets as high rate capability and high energy density storage material in hybrid supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.J.; Hou, H.S.; Banks, C.E.; Yang, Y.C.; Zhang, Y.; Ji, X.B. Alternating voltage introduced NiCo double hydroxide layered nanoflakes for an asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22741–22744. [Google Scholar] [CrossRef]
- Zheng, C.H.; Yao, T.; Xu, T.R.; Wang, H.A.; Huang, P.F.; Yan, Y.; Fang, D.L. Growth of ultrathin Ni-Co-Al layered double hydroxide on reduced graphene oxide and superb supercapacitive performance of the resulting composite. J. Alloys Compd. 2016, 678, 93–101. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Yin, Z.Y.; Wang, X.L.; Tu, J.P.; Li, J. Periodic stacking of 2D charged sheets: Self-assembled superlattice of Ni-Al layered double hydroxide (LDH) and reduced graphene oxide. Nano Energy 2016, 20, 185–193. [Google Scholar] [CrossRef]
- Li, H.B.; Gao, Y.Q.; Wang, C.X.; Yang, G.W. A simple electrochemical route to access amorphous mixed-metal hydroxides for supercapacitor electrode materials. Adv. Energy Mater. 2015, 5, 1401767. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, X.M.; Du, X.; Ma, X.L.; Hao, X.G.; Xue, C.F.; Zhu, H.Y.; Li, S.S. Controllable Synthesis of NiCo LDH nanosheets for fabrication of high-performance supercapacitor electrodes. Electroanalysis 2017, 29, 1286–1293. [Google Scholar] [CrossRef]
- Xu, J.; Ma, C.J.; Cao, J.Y.; Chen, Z.D. Facile synthesis of core-shell nanostructured hollow carbon nanospheres@nickel cobalt double hydroxides as high-performance electrode materials for supercapacitors. Dalton Trans. 2017, 46, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Q.; Shen, X.P.; Ma, L.B.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 2015, 258, 251–259. [Google Scholar] [CrossRef]
- Li, X.C.; Shen, J.J.; Sun, W.; Hong, X.D.; Wang, R.T.; Zhao, X.H.; Yan, X.B. A super-high energy density asymmetric supercapacitor based on 3D core-shell structured NiCo-layered double hydroxide@carbon nanotube and activated polyaniline-derived carbon electrodes with commercial level mass loading. J. Mater. Chem. A 2015, 3, 13244–13253. [Google Scholar] [CrossRef]
- Huang, P.; Cao, C.; Sun, Y.; Yang, S.; Wei, F.; Song, W. One-pot synthesis of sandwich-like reduced graphene oxide@CoNiAl layered double hydroxide with excellent pseudocapacitive properties. J. Mater. Chem. A 2015, 3, 10858–10863. [Google Scholar] [CrossRef]
- Song, Y.; Cai, X.; Xu, X.X.; Liu, X.X. Integration of nickel-cobalt double hydroxide nanosheets and polypyrrole films with functionalized partially exfoliated graphite for asymmetric supercapacitors with improved rate capability. J. Mater. Chem. A 2015, 3, 14712–14720. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Chen, G.F.; Zhou, P.L.; Li, N.; Su, Y.Z. Building layered NixCo2x(OH)6x nanosheets decorated three-dimensional Ni frameworks for electrochemical applications. J. Power Sources 2016, 317, 1–9. [Google Scholar] [CrossRef]
- Pu, J.; Tong, Y.; Wang, S.; Sheng, E.; Wang, Z. Nickelecobalt hydroxide nanosheets arrays on Ni foam for pseudocapacitor applications. J. Power Sources 2014, 250, 250–256. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B.C.; Hou, H.J.; Wu, L.S.; Zhu, X.L.; Hu, J.P.; Yang, J.K. Facile preparation of flower-like NiMn layered double hydroxide/reduced graphene oxide microsphere composite for high-performance asymmetric supercapacitors. J. Alloys Compd. 2018, 730, 71–80. [Google Scholar] [CrossRef]
- Chen, D.M.; Chen, H.Y.; Chang, X.; Liu, P.; Zhao, Z.C.; Zhou, J.W.; Xu, G.W.; Lin, H.L.; Han, S. Hierarchical CoMn-layered double hydroxide nanowires on nickel foam as electrode material for high-capacitance supercapacitor. J. Alloys Compd. 2017, 729, 866–873. [Google Scholar] [CrossRef]
- Li, X.Y.; Yu, L.; Wang, G.L.; Wan, G.P.; Peng, X.G.; Wang, K.; Wang, G.Z. Hierarchical NiAl LDH nanotubes constructed via atomic layer deposition assisted method for high performance supercapacitors. Electrochim. Acta 2017, 255, 15–22. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, Q.; Liu, J.Y.; Li, R.M.; Zhang, H.S.; Chen, R.R.; Wang, J. Hierarchical NiCo2O4@NiCoAl-layered double hydroxide core/shell nanoforest arrays as advanced electrodes for high-performance asymmetric supercapacitors. J. Alloys Compd. 2017, 724, 130–138. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.L.; Wu, F.; Hu, B.S.; Meng, F.M.; Niu, K.Y.; Lin, F.; Zheng, H.M. An investigation of ultrathin nickel-iron layered double hydroxide nanosheets grown on nickel foam for high-performance supercapacitor electrodes. J. Alloys Compd. 2017, 714, 63–70. [Google Scholar] [CrossRef]
- Wu, N.S.; Low, J.X.; Liu, T.; Yu, J.G.; Cao, S.W. Hierarchical hollow cages of Mn-Co layered double hydroxide as supercapacitor electrode materials. Appl. Surf. Sci. 2017, 413, 35–40. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.C.; Li, Z.H.; Sun, P.P.; Liu, F.; Dong, C.; Wang, J.S.; Li, Z.J.; Wu, M.W.; Zhang, C.; et al. Synthesis of delaminated layered double hydroxides and their assembly with graphene oxide for supercapacitor application. J. Alloys Compd. 2017, 711, 31–41. [Google Scholar] [CrossRef]
- Li, S.S.; Cheng, P.P.; Luo, J.X.; Zhou, D.; Xu, W.M.; Li, J.W.; Li, R.C.; Yuan, D.S. High-performance flexible asymmetric supercapacitor based on CoAl-LDH and rGO electrodes. Nano-Micro Lett. 2017, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Zai, J.T.; Liu, Y.Y.; Li, X.M.; Ma, Z.F.; Qi, R.R.; Qian, X.F. 3D Hierarchical Co-Al layered double hydroxides with long term stabilities and high rate performances in supercapacitors. Nano-Micro Lett. 2017, 9, 21. [Google Scholar] [CrossRef]
- Zha, D.S.; Sun, H.H.; Fu, Y.S.; Ouyang, X.P.; Wang, X. Acetate anion-intercalated nickel-cobalt layered double hydroxide nanosheets supported on Ni foam for high-performance supercapacitors with excellent long-term cycling stability. Electrochim. Acta 2017, 236, 18–27. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Zhang, X.B.; Cheng, J.P. A comparative study of Ni-Mn layered double hydroxide/carbon composites with different morphologies for supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 30068–30078. [Google Scholar] [CrossRef]
- Yuan, C.Z.; Gao, B.; Shen, L.F.; Yang, S.D.; Hao, L.; Lu, X.J.; Zhang, F.; Zhang, L.J.; Zhang, X.G. Hierarchically structured carbon-based composites: Design, synthesis and their application in electrochemical capacitors. Nanoscale 2011, 3, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, D.C.; Xiong, X.H.; Song, B.; Hu, R.Z.; Zhang, Q.B.; Rainwater, B.H.; Waller, G.H.; Zhen, D.X.; Ding, Y.; et al. A high-energy, long cycle-life hybrid supercapacitor based on graphene composite electrodes. Energy Storage Mater. 2017, 7, 32–39. [Google Scholar] [CrossRef]
- Liu, S.; Lee, S.C.; Patil, U.; Shackery, I.; Kang, S.; Zhang, K.; Park, J.H.; Chung, K.Y.; Jun, S.C. Hierarchical MnCo-layered double hydroxides@Ni(OH)2 core-shell heterostructures as advanced electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 1043–1049. [Google Scholar] [CrossRef]
- Kierzek, K.; Frackowiak, E.; Lota, G.; Gryglewicz, G.; Machnikowski, J. Electrochemical capacitors based on highly porous carbons prepared by KOH activation. Electrochim. Acta 2004, 49, 515–523. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Gong, M.; Li, Y.; Chang, W.; Mefford, T.; Zhou, J.; Wang, J.; Regier, T.; Wie, F.; et al. An ultrafast nickel-iron battery from strongly coupled inorganic nanoparticle/nanocarbon hybrid materials. Nat. Commun. 2012, 3, 917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Gong, L.Y.; Sun, K.; Jiang, J.C.; Zhang, X.G. Preparation of activated carbon from waste Camellia oleifera shell for supercapacitor application. J. Solid State Electrochem. 2012, 16, 2179–2186. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, B.; Wang, J.X.; Qu, C.; Sun, H.B.; Zhang, K.L.; Liu, M.L. High-performance hybrid supercapacitors based on self-supported 3D ultrathin porous quaternary Zn-Ni-Al-Co oxide nanosheets. Nano Energy 2016, 28, 475–785. [Google Scholar] [CrossRef]
- Liu, S.D.; Lee, S.C.; Patil, U.M.; Ray, C.; Sankar, K.V.; Zhang, K.; Kundu, A.; Kang, S.N.; Park, J.H.; Jun, S.C. Controllable sulfuration engineered NiO nanosheets with enhanced capacitance for high rate supercapacitors. J. Mater. Chem. A 2017, 5, 4543–4549. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Chen, Q.D.; Zhan, H.B. Supercapacitors based on reduced graphene oxide nanofibers supported Ni(OH)2 nanoplates with enhanced electrochemical performance. ACS Appl. Mater. Interfaces 2016, 8, 22977–22987. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.Y.; Liu, K.F.; Wang, D.W.; Zhang, H.H.; Cheng, C.J. Synthesis of hierarchical NiS microflowers for high performance asymmetric supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, B.; Hu, B.; Xu, Z.Y.; Wang, D.W.; Zhang, H.H. Vulcanizing time controlled synthesis of NiS microflowers and its application in asymmetric supercapacitors. Electrochim. Acta 2017, 230, 428–437. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Li, L.H.; Liu, L.; Xu, Y.; Ding, H.Y. Large-scale self-assembly of 3D flower-like hierarchical Ni/Co-LDHs microspheres for high-performance flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.Y.; Xie, T.; Gai, Y.S.; Su, L.H.; Gong, L.Y.; Lu, H.J.; Dong, F.Y. Self-assembled hierarchical peony-like ZnCo2O4 for high-performance asymmetric supercapacitors. Electrochim. Acta 2017, 253, 281–290. [Google Scholar] [CrossRef]
- Song, D.M.; Zhu, J.K.; Li, J.; Pu, T.; Huang, B.; Zhao, C.L.; Xie, L.; Chen, L.Y. Free-standing two-dimensional mesoporous ZnCo2O4 thin sheets consisting of 3D ultrathin nanoflake array frameworks for high performance asymmetric supercapacitor. Electrochim. Acta 2017, 257, 455–464. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Sun, C.C.; Guo, G.L.; Sun, W.P.; Huang, W.; Yan, Q.Y.; Dong, X.C. Controlled synthesis of zinc cobalt sulfide nanostructures in oil phase and their potential applications in electrochemical energy storage. J. Mater. Chem. A 2015, 3, 11462–11470. [Google Scholar] [CrossRef]
- Liu, S.; Jun, S.C. Hierarchical manganese cobalt sulfide coreeshell nanostructures for high-performance asymmetric supercapacitors. J. Power Sources 2017, 342, 629–637. [Google Scholar] [CrossRef]
- Li, S.F.; Yu, C.; Yang, J.; Zhao, C.T.; Zhang, M.D.; Huang, W.; Liu, Z.B.; Guo, W.; Qiu, J.S. A superhydrophilic “nanoglue” for stabilizing metal hydroxides onto carbon materials for high-energy and ultralong-life asymmetric supercapacitors. Energy Environ. Sci. 2017, 10, 1958–1965. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, L.J.; Zhang, H.; Zan, P.; Ge, S.; Shi, W.H.; Han, B.; Sun, H.M.; Yang, X.J.; Xu, T.H. Serpent-cactus-like Co-doped Ni(OH)2/Ni3S2 hierarchical structure composed of ultrathin nanosheets for use in efficient asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 1603–1613. [Google Scholar] [CrossRef]
- Li, W.; Xin, L.P.; Wu, M.; Long, Y.; Huang, H.T.; Lou, X.J. Facile synthesis of truncated cube-like NiSe2 single crystals for high-performance asymmetric supercapacitors. Chem. Eng. J. 2017, 330, 1334–1341. [Google Scholar]
- Shi, H. Activated carbons and double layer capacitance. Electrochim. Acta 1996, 41, 1633–1639. [Google Scholar] [CrossRef]
- Devarajan, J.; Arumugam, P. Nitrogen and phosphorous co-doped carbon nanotubes for high-performance supercapacitors. Carbon Lett. 2023, 23, 532. [Google Scholar] [CrossRef]
- Huang, X.W.; Huang, J.H.; Yang, J.; Yang, D.; Li, T.T.; Dong, A.G. High-Yield Exfoliation of Large MXene with Flake Sizes over 10 µm Using Edge-Anchored Carbon Nanotubes. Adv. Funct. Mater. 2023, 33, 2303003. [Google Scholar] [CrossRef]
- Jung, M.Y.; Sivakumar, P.; Park, H.S. Carbon nanotube branch-grown nickel nanoparticles/graphene composites for a high-capacitance electrode. J. Phys. Energy 2023, 5, 025005. [Google Scholar] [CrossRef]
- Guo, Q.H.; Li, C.W.; Yang, K.H.; Zhou, P.D.; Hua, N.B.; Weng, M.C. Polyaniline/Reduced Graphene Oxide/Carbon Nanotube Composites for Actuation-Based Sensing for Energy Storage. ACS Appl. Nano Mater. 2023, 6, 4925–4935. [Google Scholar] [CrossRef]
- Han, Y.; Ha, H.; Choi, C.; Yoon, H.; Matteini, P.; Cheong, J.Y.; Hwang, B. Review of Flexible Supercapacitors Using Carbon Nanotube-Based Electrodes. Appl. Sci. 2023, 13, 3290. [Google Scholar] [CrossRef]
- Mayank, P.; Kiran, M.S. CNT yarn based solid state linear supercapacitor with multi-featured capabilities for wearable and implantable devices. Energy Storage Mater. 2023, 57, 136–170. [Google Scholar]
- Wang, J.G.; Liu, H.Z.; Zhang, X.Y.; Li, X.; Liu, X.R.; Kang, F.Y. Green synthesis of hierarchically porous carbon nanotubes as advanced materials for high-effcient energystorage. Small 2018, 14, 1703950. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A. Carbon nanotubes-the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Chiu, C.T.; Chen, D.H. One-step hydrothermal synthesis of three-dimensional porous Ni-Co sulfide/reduced graphene oxide composite with optimal incorporation of carbon nanotubes for high performance supercapacitors. Nanotechnology 2018, 29, 175602. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, N.D.; Park, S.K.; Seong, K.D.; Hwang, M.; You, N.H.; Piao, Y.Z. Nitrogen doped carbon derived from polyimide/multiwall carbon nanotube composites for high performance flexible all-solid-state supercapacitors. J. Power Sources 2018, 380, 55–63. [Google Scholar] [CrossRef]
- Jin, L.N.; Shao, F.; Jin, C.; Zhang, J.N.; Liu, P.; Guo, M.X.; Bian, S.W. High-performance textile supercapacitor electrode materials enhanced with three-dimensional carbon nanotubes/graphene conductive network and in situ polymerized polyaniline. Electrochim. Acta 2017, 249, 387–394. [Google Scholar] [CrossRef]
- Zhou, G.H.; Kim, N.R.; Chun, S.E.; Lee, W.; Um, M.K.; Chou, T.W.; Islam, M.F.; Byun, J.H. Highly porous and easy shapeable poly-dopamine derived graphene-coated single walled carbon nanotube aerogels for stretchable wire-type supercapacitors. Carbon 2018, 130, 37–144. [Google Scholar] [CrossRef]
- Biswal, M.; Banerjee, A.; Deo, M.; Ogale, S. From dead leaves to high energy density supercapacitors. Environ. Sci. Technol. 2013, 6, 1249–1259. [Google Scholar] [CrossRef]
- Annamalai, K.; Gao, J.; Liu, L.; Mei, J.; Lau, W.; Tao, Y. Nanoporous graphene/single wall carbon nanohorn heterostructures with enhanced capacitance. J. Mater. Chem. A 2015, 3, 11740–11744. [Google Scholar] [CrossRef]
- Khaw, L.F.; Koh, W.S.; Yeap, S.P.; Lim, J.K.; Ahmad, A.L.; Toh, P.Y.; Khosravi, V. Shape-controlled synthesis of polyaniline and its synergistic effect with reduced graphene oxide for the fabrication of flexible electrode. Polym. Eng. Sci. 2023, 63, 2295–2308. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.Y.; Ma, W.H.; Wang, C.H.; Wang, X.F.; Chen, J.J.; Yu, T.T.; Fan, S. Nanocellulose/nitrogen and fluorine co-doped graphene composite hydrogels for high-performance supercapacitors. Nano Res. 2023, 23, 5736. [Google Scholar] [CrossRef]
- Dai, S.G.; Liu, Z.; Zhao, B.; Zeng, J.H.; Hu, H.; Zhang, Q.B.; Chen, D.C.; Qu, C.; Dang, D.; Liu, M.L. A high-performance supercapacitor electrode based on N-doped porous graphene. J. Power Sources 2018, 387, 43–48. [Google Scholar] [CrossRef]
- Feng, R.M.; Chen, Y.J.; Yang, L.F.; Du, Q.Z.; Zhuo, K.L. Ethyl viologen-functionalized reduced graphene oxide composites for asymmetric ionic liquid-based supercapacitors. Chem. Eng. J. 2023, 468, 143693. [Google Scholar] [CrossRef]
- Qiu, C.J.; Jiang, L.L.; Gao, Y.G.; Sheng, L.Z. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Sima, L.; Manila, O.V.; Rajinder, P.; Michael, A.P. Electrolyte-mediated assembly of graphene-based supercapacitors using adsorbed ionic liquid/non-ionic surfactant complexes. J. Mater. Chem. A 2023, 11, 11222–11234. [Google Scholar]
- Qin, Y.; Yuan, J.; Li, J.; Chen, D.C.; Kong, Y.; Chu, F.Q.; Tao, Y.X.; Liu, M.L. Crosslinking graphene oxide into robust 3D porous N-doped graphene. Adv. Mater. 2015, 27, 5171–5175. [Google Scholar] [CrossRef]
- Wang, K.L.; Xu, M.; Gu, Y.; Gu, Z.G.; Liu, J.; Fan, Q.H. Low-temperature plasma exfoliated n-doped graphene for symmetrical electrode supercapacitors. Nano Energy 2017, 31, 486–494. [Google Scholar] [CrossRef]
- Sedlovets, D.M. N-Doped Graphene-like Film/Silicon Structures as Micro-Capacitor Electrodes. Materials 2023, 16, 4007. [Google Scholar] [CrossRef]
- Lee, D.; Banda, H.; Périé, S.; Marcucci, C.; Chenavier, Y.; Dubois, L.; Taberna, P.L.; Simon, P.; Paëpe, G.D.; Duclairoir, F. Revealing Electrolytic Ion Sorption in Layered Graphene Galleries through Low-Temperature Solid-State. NMR Chem. Mater. 2023, 35, 3841–3848. [Google Scholar] [CrossRef]
- Qiu, Z.P.; Liu, Z.; Lu, X.L.; Zhang, S.; Yan, Y.C.; Chi, C.L.; Chao, H.F.; Wang, G.W.; Gao, P.F.; Chi, W.H.; et al. Dual Molecules Cooperatively Confined In-Between Edge-oxygen-rich Graphene Sheets as Ultrahigh Rate and Stable Electrodes for Supercapacitors. Small 2023, 19, e2302316. [Google Scholar] [CrossRef]
- Peng, X.Q.; Du, Y.P.; Gu, Z.; Deng, K.; Liu, X.S.; Lv, X.B.; Tian, W.; Ji, J.Y. Rearrangement of GO nanosheets with inner and outer forces under high-speed spin for supercapacitor. J. Colloid Interface Sci. 2023, 644, 167–176. [Google Scholar] [CrossRef]
- Cung, D.; Nguyen, T.; Mai, V.D.; Vu, V.P.; Kim, S.; Lee, S.H. Integrated solar-rechargeable supercapacitors with a dual-functional-layered electrode. J. Power Sources 2023, 572, 233086. [Google Scholar]
- Pang, J.; Li, J.; Guo, J.H.; Jia, M.J.; Zhang, J.W. Tuning the aggregation structure and surface composition of reduced graphene oxide microspheres for high-rate supercapacitors. Diam. Relat. Mater. 2023, 136, 109920. [Google Scholar] [CrossRef]
- Li, Z.; Huang, T.Q.; Gao, W.W.; Xu, Z.; Chang, D.; Zhang, C.X.; Gao, C. Hydrothermally activated graphene fiber fabrics for textile electrodes of supercapacitors. ACS Nano 2017, 11, 11056–11065. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, L.L.; Sheng, L.Z.; Zhou, Q.H.; Wei, T.; Zhang, B.S.; Fan, Z.J. Oxygen clusters distributed in graphene with “Paddy Land” structure: Ultrahigh capacitance and rate performance for supercapacitors. Adv. Funct. Mater. 2018, 28, 1705258. [Google Scholar] [CrossRef]
- Hang, W.L.; Xu, C.; Ma, C.Q.; Li, G.X.; Wang, Y.Z.; Zhang, K.Y.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y.W.; et al. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar]
- Zhang, W.Y.; Liu, H.L.; Kang, H.W.; Zhang, S.R.; Yang, B.C.; Li, Z.K. 2-aminoanthraquinone anchored on N-doped reduced graphene oxide for symmetric supercapacitor with boosting energy density. Electrochim. Acta 2023, 448, 142194. [Google Scholar] [CrossRef]
- Liang, J.Y.; Wang, Z.; Huang, L.T.; Zou, P.; Liu, X.L.; Ni, Q.; Wang, X.Y.; Wang, W.J.; Tao, R.M. Facile and Tunable Synthesis of Nitrogen-Doped Graphene with Different Microstructures for High-Performance Supercapacitors. ACS Mater. Lett. 2023, 5, 944–954. [Google Scholar] [CrossRef]
- Song, B.; Zhao, J.X.; Wang, M.J.; Mullaveya, J.; Zhua, Y.T.; Geng, Z.S.; Chen, D.C.; Ding, Y.; Moon, K.; Liu, M.L.; et al. Systematic study on structural and electronic properties of diamine/triamine functionalized graphene networks for supercapacitor application. Nano Energy 2017, 31, 183–193. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, L.N.; Dong, H.Z.; He, W.B.; Dong, L.F.; Yu, L.Y. High-Performance Supercapacitor of Graphene Quantum Dots with Uniform Sizes. ACS Appl. Mater. Interfaces 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.L.; El-Kady, M.F.; Lin, C.W.; Zhu, G.Z.; Marsh, K.L.; Hwang, J.Y.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z.; Kaner, R.B. 3D freeze-casting of cellular graphene films for ultrahigh power-density supercapacitors. Adv. Mater. 2016, 28, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Song, B.; Maurer, L.; Lin, Z.Y.; Lian, G.; Tuan, C.C.; Moon, K.S.; Wong, C.P. Molecular engineering of aromatic amine spacers for high-performance graphene-based supercapacitors. Nano Energy 2016, 21, 276–294. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, F.; Zhang, T.; Leng, K.; Zhang, L.; Yang, X.; Ma, Y.; Huang, Y.; Zhang, M.; Chen, Y. Synthesis and supercapacitor performance studies of N-dope graphene materials using o-phenylenediamine as the double-N precursor. Carbon 2013, 63, 508–516. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, L.; Kang, H.Q.; Dong, H.Z.; Dong, L.F.; Yu, L.Y. High performance of N-doped graphene with bubble-like textures for supercapacitors. Small 2018, 14, 1702570. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Huang, S.F.; Xia, M.R.; Rehman, S.; Mu, S.C.; Kou, Z.K.; Zhang, Z.; Chen, Z.Y.; Gao, F.M.; Hou, Y.L. N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted “cutting-thin” technique: A universal synthesis and multifunctional applications. Nano Energy 2016, 28, 346–355. [Google Scholar] [CrossRef]
- Zhao, C.M.; Zheng, W.T. A review for aqueous electrochemical supercapacitors. Front. Energy Res. 2015, 3, 23. [Google Scholar] [CrossRef]
- Amaral, M.M.; Venâncio, R.; Peterlevitz, A.C.; Zanin, H. Recent advances on quasi-solid-state electrolytes for supercapacitors. J. Energy Chem. 2021, 11, 11. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.H.; Liu, W.; Liu, H.Y.; Dai, L.; Zhang, X.Y.; Si, C.L.; Du, H.H.; et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater. 2022, 3, 13. [Google Scholar] [CrossRef]
- Qu, J.Q.; Bai, Y.C.; Li, X.M.; Song, K.L.; Zhang, S.; Wang, X.X.; Wang, X.C.; Dai, S.G. Rational design of NiSe2@rGO nanocomposites for advanced hybrid supercapacitors. J. Mater. Res. Technol. 2021, 15, 6155–6161. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.J.; Yuan, X.C.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q.D. A high-energy density asymmetric supercapacitor based on Fe2O3 nanoneedle arrays and NiCo2O4/Ni(OH)2 hybrid nanosheet arrays grown on SiC nanowire networks as free-standing advanced electrodes. Adv. Energy Mater. 2018, 8, 1702787. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Liu, Z.C.; Zhao, B.; Cheng, Y.; Zhang, L.; Wu, H.H.; Wang, M.S.; Dai, S.G.; Zhang, K.L.; Ding, D.; et al. Design and understanding of dendritic mixed-metal hydroxide nanosheets@N-doped carbon nanotube array electrode for highperformance asymmetric supercapacitors. Energy Storage Mater. 2018, 16, 632–645. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.L.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J.Y. Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef]
- Chang, X.W.; Liu, T.T.; Li, W.L.; Gao, R.X.; Lei, H.; Ren, Z.Y. Porous prussian blue analogs derived nickel-iron bimetallic phosphide nanocubes on conductive hollow mesoporous carbon nanospheres for stable and flexible high-performance supercapacitor electrode. J. Colloid Interface Sci. 2023, 650, 728–741. [Google Scholar] [CrossRef]
- Ji, P.Y.; Li, Q.Y.; Zhang, X.M.; Hu, Y.W.; Han, X.Y.; Zhang, D.Z.; Hu, C.G.; Xi, Y. Achieving Continuous Self-Powered Energy Conversion-Storage-Supply Integrated System Based on Carbon Felt. Adv. Sci. 2023, 10, 2207033. [Google Scholar]
- Kim, S.I.; Kang, J.H.; Kim, S.W.; Jang, J.H. A new approach to high-performance flexible supercapacitors: Mesoporous three-dimensional Ni-electrodes. Nano Energy 2017, 39, 639–646. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Cai, X.Y.; Qian, Y.; Jiang, H.F.; Zhou, L.J.; Li, B.S.; Lai, L.F.; Shen, Z.X.; Huang, W. Electrochemically synthesis of nickel cobalt sulfde for high-performance flexible asymmetric supercapacitors. Adv. Sci. 2018, 5, 1700375. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wei, J.C.; Xiao, Y.B.; Xu, Y.Z.; Xiao, Y.J.; Wang, Y.; Tan, L.C.; Yuan, K.; Chen, Y.W. When Al-doped cobalt sulfide nanosheets meet nickel nanotube arrays: A highly efficient and stable cathode for asymmetric supercapacitors. ACS Nano 2018, 12, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hwang, J.; Kim, D.; Ahn, H. Oxygen incorporated in 1T/2H hybrid MoS2 nanoflowers prepared from molybdenum blue solution for asymmetric supercapacitor applications. Chem. Eng. J. 2021, 419, 129701. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Hamideh, M.S.; Hamidreza, N.; Fatemeh, F.; Fariba, S. Fabrication of a high-performance hybrid supercapacitor using a modified graphene aerogel/cerium oxide nanoparticle composite. J. Energy Storage 2019, 26, 100998. [Google Scholar]
- Nagaraju, G.; Sekhar, S.C.; Bharat, L.K.; Yu, J.S. Wearable fabrics with self-branched bimetallic layered double hydroxide coaxial nanostructures for hybrid supercapacitors. ACS Nano 2017, 11, 10860–10874. [Google Scholar] [CrossRef]
- Lee, D.G.; Choi, H.; Park, Y.; Kim, M.C.; Park, J.B.; Lee, S.; Cho, Y.Y.; Ahn, W.; Jang, A.R.; Sohn, J.I.; et al. Utilizing hybrid faradaic mechanism via catalytic and surface interactions for high-performance flexible energy storage system. J. Energy Chem. 2023, 83, 541–548. [Google Scholar] [CrossRef]
- Dai, S.G.; Liu, J.L.; Wang, C.S.; Wang, X.; Xi, Y.; Wei, D.P.; Hu, C. Hierarchical porous nanostructures of manganese(III) oxyhydroxide for all-solid-state flexible supercapacitors. Energy Technol. 2016, 4, 1450–1454. [Google Scholar] [CrossRef]
- Kareri, T.; Hossain, M.S.; Ram, M.K.; Takshi, A. A flexible fiber-shaped hybrid cell with a photoactive gel electrolyte for concurrent solar energy harvesting and charge storage. Energy Res. 2022, 46, 17084–17095. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Sheng, L.Z.; Lin, Y.Q.; Zhang, S.; Zhang, L.H.; Wei, T.; Zhou, J.L.; Liu, C.Q.; Jiang, H.; Zhou, Q.; et al. Weldable and flexible graphene ribbon@Ni fibers with ultrahigh length capacitancefor all-solid-state supercapacitors. Chem. Eng. J. 2021, 426, 131361. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.C.; Wang, X.N.; Zhao, J.X.; Guo, J.B.; Zhou, Z.Y.; Zhang, J.; Man, P.; Sun, J.; Li, Q.W.; et al. Constructing hierarchical dandelion-like molybdenum-nickel-cobalt ternary oxide nanowire arrays on carbon nanotube fiber for highperformance wearable fiber-shaped asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 21153–21160. [Google Scholar] [CrossRef]
- Jin, H.Y.; Zhou, L.M.; Mak, C.L.; Huang, H.T.; Tang, W.M.; Chan, H.L. High-performance fiber-shaped supercapacitors using carbon fiber thread (CFT)@polyanilne and functionalized CFT electrodes for wearable/stretchable electronics. Nano Energy 2015, 11, 662–670. [Google Scholar] [CrossRef]
- Liu, W.W.; Feng, K.; Zhang, Y.N.; Yu, T.W.; Han, L.; Lui, G.; Lia, M.; Chiu, G.; Fung, P.; Yu, A.P. Hair-based flexible knittable supercapacitor with wide operating voltage and ultra-high rate capability. Nano Energy 2017, 34, 491–499. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Kim, J.; Wang, Y.; Huang, H.T.; Kim, Y.S. Flexible and wearable fiber shaped high voltage supercapacitors based on copper hexacyanoferrate and porous carbon coated carbon fiber electrodes. J. Mater. Chem. A 2016, 4, 493440. [Google Scholar] [CrossRef]
- Dai, S.G.; Guo, H.Y.; Wang, M.J.; Liu, J.L.; Wang, G.; Hu, C.G.; Xi, Y. A Flexible micro-supercapacitor based on a pen ink-carbon fiber thread. J. Mater. Chem. A 2014, 2, 19665–19669. [Google Scholar] [CrossRef]
- Lee, J.; Shin, M.K.; Kim, S.H.; Cho, H.U.; Spinks, G.M.; Wallace, G.G.; Lima, M.D.; Lepro, X.; Kozlov, M.; Baughman, R.H.; et al. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 2013, 4, 1970. [Google Scholar] [CrossRef]
- Kou, L.; Huang, T.; Zheng, B.; Han, Y.; Zhao, X.; Gopalsamy, K.; Sun, H.; Gao, C. Coaxial wet-spun yarn supercapacitors for high-energy density and safe wearable electronics. Nat. Commun. 2014, 5, 3754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Xu, W.W.; Sun, J.; Pan, Z.H.; Zhao, J.X.; Wang, X.N.; Zhang, J.; Man, P.; Guo, J.B.; Zhou, Z.Y.; et al. Constructing ultrahigh-capacity zinc-nickel-cobalt oxide@Ni(OH)2 core-shell nanowire arrays for high-performance coaxial fiber shaped asymmetric supercapacitors. Nano Lett. 2017, 17, 7552–7560. [Google Scholar] [CrossRef]
- Li, Z.H.; Shaon, M.F.; Zhou, L.; Zhang, R.K.; Zhang, C.; Han, J.B.; Wein, M.; Evans, D.G.; Duan, X. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@layered double hydroxide core-shell nanoarrays. Nano Energy 2016, 20, 294–304. [Google Scholar] [CrossRef]
- Nagaraju, G.; Sekhar, S.C.; Yu, J.S. Utilizing waste cable wires for high-performance fiber-based hybrid supercapacitors: An effective approach to electronic-waste management. Adv. Energy Mater. 2018, 8, 1702201. [Google Scholar] [CrossRef]
- Gao, L.B.; Surjad, J.U.; Cao, K.; Zhang, H.T.; Li, P.F.; Xu, S.; Jiang, C.C.; Song, J.; Sun, D.; Lu, Y. Flexible fiber-shaped supercapacitor based on nickel-cobalt double hydroxide and pen ink electrodes on metallized carbon fiber. ACS Appl. Mater. Interfaces 2017, 9, 5409–5418. [Google Scholar] [CrossRef]
- Varakin, I.; Stepanov, A.; Menukhov, V. Capacitor with a Double Electrical Layer. U.S. Patent 5,986,876, 14 August 1995. [Google Scholar]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Romero, P.G. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]












| Electrode Materials | Electrolyte | Voltage (V) | Current Load or Scan Rate | Specific Capacity (C g−1) | Reference |
|---|---|---|---|---|---|
| 3D nanoporous Ni(OH)2 | 6.0 M KOH | 0–0.5 | 7 A g−1 | 759.5 | [55] |
| Ni(OH)2 nanospheres | 1.0 M KOH | 0–0.5 | 20 A g−1 | 934 | [56] |
| α-Ni(OH)2 nanobristles | 1.0 M KOH | 0–0.45 | 2 A g−1 | 940.5 | [57] |
| Ni(OH)2 microspheres | 2.0 M KOH | 0–0.55 | 0.5 A g−1 | 704.5 | [58] |
| Mesoporous a-Ni(OH)2 | 2.0 M KOH | 0–0.55 | 0.5 A g−1 | 983.9 | [59] |
| Ni(OH)2 nanoboxes | 2.0 M KOH | 0–0.5 | 1 A g−1 | 1247.5 | [60] |
| α-Ni(OH)2 nanowires | 2.0 M KOH | 0–0.4 | 1 A g−1 | 889.2 | [61] |
| Ni(OH)2 nanosheets | 6.0 M KOH | 0–0.5 | 2 A g−1 | 825.6 | [62] |
| Ni(OH)2 nanoflakes | 1.0 M KOH | 0–0.4 | 1 A g−1 | 566.4 | [63] |
| Ni(OH)2 nanocubes | 3.0 M KOH | 0–0.45 | 1 A g−1 | 828.9 | [64] |
| Amorphous α-Ni(OH)2 | 2.0 M KOH | 0–0.35 | 2 A g−1 | 818.3 | [65] |
| Cabbage-like α-Ni(OH)2 | 1.0 M KOH | 0.2–0.6 | 1 mA cm−2 | 761.2 | [66] |
| Ni(OH)2 nanosheets | 2.0 M KOH | 0–0.45 | 1 A g−1 | 1072.9 | [67] |
| Ni(OH)2 platelets | 2.0 M KOH | 0–0.6 | 0.5 A g−1 | 1160.4 | [68] |
| β-Ni(OH)2 nanosheets | 6.0 M KOH | 0–0.6 | 5 mV s−1 | 1041 | [69] |
| α-Ni(OH)2 | 2.0 M KOH | 0–0.5 | 2 mV s−1 | 267 | [70] |
| α-Ni(OH)2 microspheres | 6.0 M KOH | 0–0.4 | 1 A g−1 | 992.3 | [71] |
| β-Ni(OH)2 nanosheets | 6.0 M KOH | 0–0.4 | 5 mA cm−2 | 790.3 | [72] |
| β-Ni(OH)2 | 6.0 M KOH | −0.05–0.35 | 1 A g−1 | 712 | [73] |
| Electrode Materials | ∆E (V) | Maximum Capacity (C g−1) | Capacity Retention | Cycle Stability | Ref. |
|---|---|---|---|---|---|
| Ni(OH)2 nanoplatelets/rGO | 0.45 | 955 C g−1 (1 A g−1) | 58.6% (80 A g−1) | 102% (5000 cycles) | [74] |
| 3D Ni(OH)2/rGO network | 0.5 | 563 C g−1 (0.5 A g−1) | 61.8% (10 A g−1) | 87% (1000 cycles) | [75] |
| Ni(OH)2/rGO | 0.6 | 941 C g−1 (4 A g−1) | 27% (11.2 A g−1) | 75% (1000 cycles) | [76] |
| Ni(OH)2/rGO aerogel | 0.5 | 516 C g−1 (0.5 A g−1) | 54.3% (2 A g−1) | 95% (2000 cycles) | [77] |
| Ni(OH)2/3D rGO | 0.5 | 690 C g−1 (1 A g−1) | 86.7% (60 A g−1) | 78% (1000 cycles) | [78] |
| Ni(OH)2 nanoparticles/rGO | 0.38 | 858 C g−1 (0.5 A g−1) | 52.7% (10 A g−1) | 89% (1000 cycles) | [79] |
| Ni(OH)2 nanosheets/rGO | 0.45 | 838 C g−1 (0.8 A g−1) | 62.3% (6.4 A g−1) | 92% (2000 cycles) | [80] |
| Ni(OH)2 nanocrystals/rGO | 0.5 | 951.5 C g−1 (1 A g−1) | 60.9% (20 A g−1) | 70% (1000 cycles) | [81] |
| Ni(OH)2 nanoplates/rGO | 0.5 | 667 C g−1 (2.8 A g−1) | 71% (45.7 A g−1) | 100% (2000 cycles) | [82] |
| Ni(OH)2/rGO | 0.55 | 1206 C g−1 (2 mV s−1) | 41% (20 mV s−1) | 95% (2000 cycles) | [83] |
| Ni(OH)2/rGO | 0.55 | 954 C g−1 (1 mV s-1) | 30% (50 mV s−1) | 88% (1000 cycles) | [84] |
| β-Ni(OH)2/rGO | 0.5 | 971 C g−1 (1 A g-1) | 67.9% (40 A g−1) | 81% (2000 cycles) | [85] |
| Ni(OH)2 nanoflowers/rGO | 0.55 | 598 C g−1 (1 A g-1) | 58% (10 A g−1) | 95% (1000 cycles) | [86] |
| Flower-like Ni(OH)2/rGO | 0.4 | 642 C g−1 (1 A g-1) | 18.7% (30 A g−1) | 86% (2200 cycles) | [87] |
| Ni(OH)2/rGO | 0.45 | 546 C g−1 (5 mV s-1) | 35% (100 mV s−1) | 88% (1000 cycles) | [88] |
| Electrode Materials | ∆E (V) | Maximum Capacity (C g−1) | Capacity Retention | Cycle Stability | Ref. |
|---|---|---|---|---|---|
| NiCo-LDH/CC | 0.5 | 908 C g−1 (1 A g−1) | 60% (100 A g−1) | 88% (10000 cycles) | [177] |
| NiCo-LDH/CFC | 0.45 | 1009 C g−1 (1 A g−1) | 61% (60 A g−1) | 95% (2000 cycles) | [178] |
| NiCoAl-LDH | 0.6 | 1237 C g−1 (1 A g−1) | 48.7% (10 A g−1) | 93% (3000 cycles) | [179] |
| NiCoAl-LDH@BG-NF | 0.5 | 999 C g−1 (6 A g−1) | 75.3% (20 A g−1) | 91% (2000 cycles) | [180] |
| rGO/Ni0.75−xCoxAl0.25-LDH | 0.5 | 772 C g−1 (1 A g−1) | 70% (40 A g−1) | 89% (2000 cycles) | [181] |
| MnCo-LDH@Ni(OH)2 | 0.4 | 928 C g−1 (1 A g−1) | 56.3% (30 A g−1) | 91% (5000 cycles) | [182] |
| Ni0.76Co0.24-LDH | 0.5 | 1279 C g−1 (1 A g−1) | 87.2% (30 A g−1) | 70% (20000 cycles) | [183] |
| HCNs@NiCo-LDH | 0.5 | 1095 C g−1 (1 A g−1) | 74.9% (20 A g−1) | — | [184] |
| NiCo-LDH/rGO | 0.45 | 802 C g−1 (1 A g−1) | 76.9% (20 A g−1) | 74% (1000 cycles) | [185] |
| NiCo-LDH@CNT/NF | 0.4 | 818 C g− 1(1 A g−1) | 65.2% (20 A g−1) | — | [186] |
| rGO(25)@CoNiAl-LDH | 0.45 | 839.7 C g−1 (1 A g−1) | 72.9% (10 A g−1) | 100% (5000 cycles) | [187] |
| LEG/NiCo-LDH | 0.45 | 1099 C g−1 (1 A g−1) | 83.5% (50 A g−1) | 89% (5000 cycles) | [188] |
| NixCo2x(OH)6x@Ni | 0.5 | 1146 C g−1 (5 A g−1) | 68% (100 A g−1) | 90% (5000 cycles) | [189] |
| NiCo-LDHs | 0.45 | 780 C g−1 (6 A g−1) | 66.1% (30 A g−1) | 86% (1000 cycles) | [173] |
| Ni-Co LDH NSs/CTs | 0.5 | 1052 C g−1 (2 A g−1) | 57% (20 A g−1) | 90% (2000 cycles) | [190] |
| NiMn LDH/rGO | 0.5 | 750 C g−1 (1 A g−1) | 45.3% (10 A g−1) | 90% (5000 cycles) | [191] |
| CoMn-LDH | 0.65 | 916 C g−1 (1 A g−1) | 71.1% (10 A g−1) | 93% (3000 cycles) | [192] |
| 10NiAl-LDH | 0.4 | 849 C g−1 (0.5 A g−1) | 70.7% (20 A g−1) | 92% (10,000 cycles) | [193] |
| NiCo2O4@NiCoAl-LDH | 0.6 | 1088 C g−1 (1 A g−1) | 61% (20 A g−1) | 93% (2000 cycles) | [175] |
| Ni-Co LDH/graphene | 0.6 | 759 C g−1 (1 A g−1) | 50% (10 A g−1) | 93% (2000 cycles) | [165] |
| NiAl-LDH | 0.55 | 1023.8 C g−1 (2 A g−1) | 68.8% (50 A g−1) | 86% (5000 cycles) | [194] |
| NiFe-LDH | 0.4 | 1354 C g−1 (5 A g−1) | 53.7% (10 A g−1) | 43% (500 cycles) | [195] |
| MnCo-LDH | 0.45 | 230 C g−1 (1 A g−1) | 69.7% (20 A g−1) | 92% (2000 cycles) | [196] |
| GO/NiAl-LDHs | 0.4 | 384 C g−1 (1 A g−1) | 67% (10 A g−1) | 70% (2000 cycles) | [197] |
| NiAl-LDHs | 0.45 | 277.6 C g−1 (1 A g−1) | 73.6% (20 A g−1) | 96% (2000 cycles) | [198] |
| Co-Al-LDH | 0.45 | 377 C g−1 (1 A g−1) | 80.8% (100 A g−1) | 95% (20,000 cycles) | [199] |
| A-NiCo-LDHs | 0.5 | 1224 C g−1 (1 A g−1) | 67.1% (20 A g−1) | 93% (10,000 cycles) | [176] |
| Ni-Co@Ni-Co LDH | 0.5 | 1207 C g−1 (1 A g−1) | 82.1% (20 A g−1) | 98% (5000 cycles) | [200] |
| Ni-Mn LDH/carbon | 0.5 | 634 C g−1 (1 A g−1) | 78.4% (10 A g−1) | 79% (5000 cycles) | [201] |
| Device | Voltage (V) | Energy Density (Wh kg−1) | Power Density (W kg−1) | Cycle Performance | Ref. |
|---|---|---|---|---|---|
| Zn-Ni-Al-Co oxide//AC | 0–1.5 | 72.4 | 533 | 90% (10,000 cycles) | [207] |
| NiO/Ni3S2//AC | 0–1.6 | 52.9 | 1600 | 92.9% (5000 cycles) | [208] |
| Ni(OH)2//AC | 0–1.3 | 35.7 | 490 | 81% (10,000 cycles) | [57] |
| Ni(OH)2//AC | 0–1.6 | 22 | 800 | 85.7% (4000 cycles) | [67] |
| Ni(OH)2-AB//AC | 0–1.4 | 18.7 | 1971 | 91% (5000 cycles) | [69] |
| β-Ni(OH)2//AC | 0–1.6 | 36.2 | 100.6 | 92% (1000 cycles) | [200] |
| rGONF/Ni(OH)2//AC | 0–1.7 | 44.1 | 467 | 77.4% (2000 cycles) | [209] |
| NiS//AC | 0–1.8 | 31 | 900 | 100% (1000 cycles) | [210] |
| NiS//AC | 0–1.6 | 33.4 | 800 | 87.3% (5000 cycles) | [211] |
| Ni/Co-LDHs//AC | 0–1.6 | 165.5 | 1530 | 85% (500 cycles) | [212] |
| ZnCo2O4//AC | 0–1.6 | 29.7 | 398.5 | 72.5% (1000 cycles) | [213] |
| ZnCo2O4//AC | 0–1.6 | 33.98 | 800 | 93.3% (10,000 cycles) | [214] |
| NiCo2O4/rGO//AC | 0–1.5 | 57 | 375 | 90.2% (20,000 cycles) | [137] |
| NiCo2S4/Co9S8//AC | 0–1.5 | 33.5 | 150 | 65% (5000 cycles) | [144] |
| CuCo2S4-HNN//AC | 0–1.6 | 44.1 | 800 | 94.1% (6000 cycles) | [215] |
| MCS/GNF//AC | 0–1.6 | 54.26 | 1120 | 81.9% (4000 cycles) | [216] |
| NiCo2S4//AC | 0–1.6 | 25.5 | 334 | 93.4% (1500 cycles) | [168] |
| NiCo2S4//AC | 0–1.6 | 42.7 | 1583 | 92% (10,000 cycles) | [170] |
| NiCo2S4 nanopetals//AC | 0–1.6 | 35.6 | 819.5 | 94.3% (5000 cycles) | [217] |
| NiCo-LDH//AC | 0–1.6 | 69.7 | 800 | 87% (20,000 cycles) | [177] |
| NiCo-LDH//AC | 0–1.5 | 17.5 | 10500 | 91.2% (10,000 cycles) | [167] |
| MnCo-LDH@Ni(OH)2//AC | 0–1.5 | 47.9 | 750.7 | 90.9% (5000 cycles) | [182] |
| NiCo-LDH//AC | 0–0.8 | 15.9 | 400 | 82.7% (20,000 cycles) | [183] |
| NiCo2O4@NiCoAl-LDH//AC | 0–1.6 | 74.6 | 800 | 93% (2000 cycles) | [175] |
| NiCo-LDH/graphene//AC | 0–1.7 | 68 | 594.9 | 94.2% (2500 cycles) | [165] |
| NiFe-LDH//AC | 0–1.6 | 50.2 | 800 | 65% (2000 cycles) | [195] |
| NiMoO4//AC | 0–1.7 | 60.9 | 850 | 85.7% (10,000 cycles) | [218] |
| NiCo-10//AC | 0–1.6 | 51.5 | 825 | 89.5% (6000 cycles) | [219] |
| NiSe2//AC | 0–1.6 | 44.8 | 969.7 | 87.4% (20,000 cycles) | [220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Lin, Z.; Wang, X.; Zhang, K.; Hu, H.; Dai, S. Electrode Materials, Structural Design, and Storage Mechanisms in Hybrid Supercapacitors. Molecules 2023, 28, 6432. https://doi.org/10.3390/molecules28176432
Du X, Lin Z, Wang X, Zhang K, Hu H, Dai S. Electrode Materials, Structural Design, and Storage Mechanisms in Hybrid Supercapacitors. Molecules. 2023; 28(17):6432. https://doi.org/10.3390/molecules28176432
Chicago/Turabian StyleDu, Xiaobing, Zhuanglong Lin, Xiaoxia Wang, Kaiyou Zhang, Hao Hu, and Shuge Dai. 2023. "Electrode Materials, Structural Design, and Storage Mechanisms in Hybrid Supercapacitors" Molecules 28, no. 17: 6432. https://doi.org/10.3390/molecules28176432
APA StyleDu, X., Lin, Z., Wang, X., Zhang, K., Hu, H., & Dai, S. (2023). Electrode Materials, Structural Design, and Storage Mechanisms in Hybrid Supercapacitors. Molecules, 28(17), 6432. https://doi.org/10.3390/molecules28176432