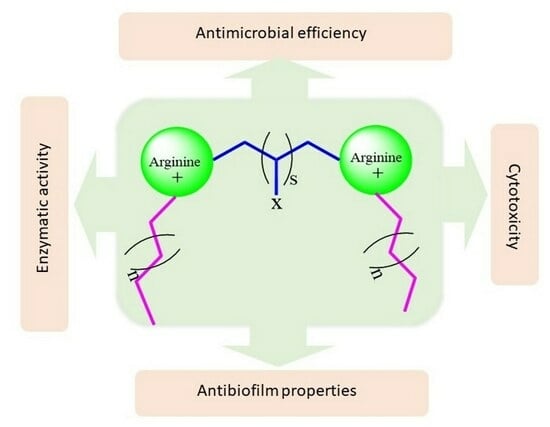
Arginine Gemini-Based Surfactants for Antimicrobial and Antibiofilm Applications: Molecular Interactions, Skin-Related Anti-Enzymatic Activity and Cytotoxicity
Abstract
:1. Introduction
2. Result and Discussion
2.1. Ionization State
2.2. Antimicrobial Activity
Antibiofilm Activity
2.3. Surface Pressure Molecular Area Isotherms and Elastic Modulus
2.3.1. Langmuir Isotherms
2.3.2. Mechanical Properties of the Mixed Monolayers
2.4. Anti-Enzymatic Inhibitory Activities
2.5. Molecular Docking Results
2.6. Cytotoxicity
3. Materials and Methods
3.1. Materials
Synthesis of Gemini Arginine Surfactants
3.2. Determination of pKa
3.3. Monolayer Isotherms
3.4. Antimicrobial Activity
Antibiofilm Activity
3.5. Anti-Enzymatic Activities
3.5.1. Collagenase Inhibitory Activity Assay
3.5.2. Elastase Inhibitory Assay
3.5.3. Hyaluronidase Inhibitory Activity
3.6. Molecular Docking Materials
3.7. Cell Culture
3.7.1. Cell Viability Assays
3.7.2. NRU Assay
3.7.3. MTT Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Yadav, M.K.; Song, J.-J.; Singh, B.P.; Vidal, J.E. Microbial biofilms and human disease: A concise review. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2015, 15, 599–607. [Google Scholar] [CrossRef]
- Piechota, M.; Kot, B.; Frankowska-Maciejewska, A.; Gruzewska, A.; Woźniak-Kosek, A. Biofilm Formation by Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus strains from hospitalized patients in Poland. Biomed. Res. Int. 2018, 2018, 4657396. [Google Scholar] [CrossRef]
- Zana, R.; Xia, J. (Eds.) Gemini Surfactants, Synthesis, Interfacial and Solution-Phase Behabiour and Applications; Marcel Dekker Inc.: New York, NY, USA, 2014; ISBN 0-8247-4705-4. [Google Scholar]
- Brycki, B.; Szulc, A. Gemini Alkyldeoxy-D-Glucitolammonium Salts as Modern Surfactants and Microbiocides: Synthesis, Antimicrobial and Surface Activity, Biodegradation. PLoS ONE 2014, 9, e84936. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Vijayasarathy, P.; Sin, A.; Nam, H.; Khan, S.; ParamEbath, J.B.M.; Mohamed, A.A.; Han, C. Antimicrobial activity of quaternary ammonium salts: Structure-activity relationship. Med. Chem. Res. 2022, 31, 1663–1678. [Google Scholar] [CrossRef]
- Jennings, M.C.; Ator, L.E.; Paniak, T.J.; Minbiole, K.P.C.; Wuest, W.M. Biofilm-Eradicating Properties of Quaternary Ammonium Amphiphiles: Simple Mimics of Antimicrobial Peptides. ChemBioChem 2014, 15, 2211–2215. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? Chem. Med. Chem. 2012, 7, 22–31. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Pons, R.; Infante, M.R. Gemini surfactants from natural amino acids. Adv. Colloid Interface Sci. 2014, 205, 134–155. [Google Scholar] [CrossRef]
- Peters, B.J.; Van Cleave, C.; Haase, A.A.; Hough, J.P.; Giffen-Kent, K.A.; Cardiff, G.M.; Sostarecz, A.G.; Crick, D.C.; Crans, D.C. Structure Dependence of Pyridine and Benzene Derivatives on Interactions with Model Membranes. Langmuir 2018, 34, 8939–8951. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Wen, X.; Pérez, L.; Infante, M.R.; Franses, E.I. Aggregation Behavior in Water of Monomeric and Gemini Cationic Surfactants Derived from Arginine. Langmuir 1999, 15, 3134–3142. [Google Scholar] [CrossRef]
- Fitch, C.A.; Platzer, G.; Okon, M.; Garcia-Moreno, B.E.; Mcintoch, L.P. Arginine: Its pKa value revisited. Protein Sci. 2015, 24, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, M.P.; Infante, M.R. Green amino acid-based surfactants. Green Chem. 2004, 6, 233–240. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino acid–based surfactants: New antimicrobial agents. Adv. Colloid. Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.N.; Zhanel, G.G.; Scheweizer, F. Antibacterial activity of ultrashort cationic lipo-β-peptides. Antimicrob. Agents Chemother. 2009, 53, 2215–2217. [Google Scholar] [CrossRef]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharm. 2013, 67, 456–467. [Google Scholar] [CrossRef]
- Obłąk, E.; Futoma-Kołoch, B.; Wieczyńska, A. Biological activity of quaternary ammonium salts and resistance of microorganisms to these compounds. World J. Microbiol. Biotechnol. 2021, 37, 22. [Google Scholar] [CrossRef]
- Pérez, L.; García, M.T.; Ribosa, I.; Vinardell, M.P.; Manresa, A.; Infante, M.R. Biological properties of arginine-based gemini cationic surfactants. Environ. Toxicol. Chem. 2002, 21, 1279–1285. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid. Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Li, L.; Vorobyov, I.; Allen, T.W. The Different Interactions of Lysine and Arginine Side Chains with Lipid Membranes. J. Phys. Chem. B 2013, 117, 11906–11920. [Google Scholar] [CrossRef] [PubMed]
- Haldar, J.; Kondaiah, P.; Bhattacharya, S. Synthesis and Antibacterial Properties of Novel Hydrolyzable Cationic Amphiphiles. Incorporation of Multiple Head Groups Leads to Impressive Antibacterial Activity. J. Med. Chem. 2005, 48, 3823–3831. [Google Scholar] [CrossRef]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, A.; Morán, C.; Raluy, M.; Pérez, L. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liq. 2019, 289, 111156. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, In Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- García, M.T.; Bautista, E.; de la Fuente, A.; Pérez, L. Cholinium-Based Ionic Liquids as Promising Antimicrobial Agents in Pharmaceutical Applications: Surface Activity, Antibacterial Activity and Ecotoxicological Profile. Pharmaceutics 2023, 15, 1806. [Google Scholar] [CrossRef]
- Koziróg, A.; Kregiel, D.; Brycki, B. Action of Monomeric/Gemini Surfactants on Free Cells and Biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Lopez, V.; Martinez-Suárez, J.V. The influence of subminimal inhibitory concentrations of benzalkonium chloride on biofilm formation by Listeria onocytogenes. Int. J. Food Microbiol. 2014, 189, 106–112. [Google Scholar] [CrossRef]
- Cabo, M.L.; Herrera, J.J.; Crespo, M.D.; Pastoriza, L. Comparison among the effectiveness of ozone, nisin and benzalkonium chloride for the elimination of planktonic cells and biofilms of Staphylococcus aureus CECT4459 on polypropylene. Food Control 2009, 20, 521–525. [Google Scholar] [CrossRef]
- Taek-Seung, K.; Hee-Deung, P. Lauroyl arginate ethyl: An effective antifouling agent applicable for reverse osmosis processes producing potable water. J. Membr. Sci. 2016, 507, 24–33. [Google Scholar]
- Rodrigues de Almeida, N.; Han, Y.; Perez, J.; Kirkpatrick, S.; Wang, Y.; Sheridan, M.C. Design, Synthesis, and Nanostructure-Dependent Antibacterial Activity of Cationic Peptide Amphiphiles. ACS Appl. Mater. Interfaces 2019, 11, 2790–2801. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Guz-Regner, K.; Dworniczek, E. Antibacterial activity of gemini quaternary ammonium salts. Microbiol. Lett. 2014, 350, 190–198. [Google Scholar] [CrossRef]
- Piecuch, A.; Obłąk, E.; Guz-Antibacterial, K. Activity of Alanine-Derived Gemini Quaternary Ammonium Compounds. J. Surfactants Deterg. 2016, 19, 275–282. [Google Scholar] [CrossRef]
- McConlogue, C.W.; Malamud, D.; Vanderlick, T.K. Interaction of DPPC monolayers with soluble surfactants: Electrostatic effects of membrane perturbants. Biochim. Biophys. Acta Biomembr. 1998, 1372, 124–134. [Google Scholar] [CrossRef]
- Pérez, L.; Sentís, A.; Hafidi, Z.; Pinazo, A.; García, M.T.; Martín-Pastor, M.; Oliveira de Sousa, F.F. Zein Nanoparticles containing Arginine-based Surfactants: Physicochemical characterization and effect on the Biological properties. Int. J. Mol. Sci. 2023, 24, 2568. [Google Scholar] [CrossRef]
- Birdi, K.S. Lipid and Biopolymer Monolayers at Liquid Interfaces; Plenum Press: New York, NY, USA, 1989; ISBN 101489925279. [Google Scholar]
- Stefaniu, C.; Brezesinski, G.; Möhwald, H. Langmuir monolayers as models to study processes at membrane surfaces. Adv. Colloid Interf. Sci. 2014, 208, 197–213. [Google Scholar] [CrossRef]
- Krigel, J.; Li, J.B.; Miller, R.; Bree, M.; Kretzschmar, G.; Möhwald, H. Surface viscoelasticity of phospholipid monolayers at the air/water interface. Colloid Polym. Sci. 1996, 274, 1183–1187. [Google Scholar] [CrossRef]
- Colomer, A.; Perez, L.; Pons, R.; In-fante, M.R.; Perez-Clos, D.; Manresa, A.; Espuny, M.; Pinazo, A. Mixed Monolayer of DPPC and Lysine-Based Cationic Surfactants: An Investigation into the Antimicrobial Activity. Langmuir 2013, 29, 7912–7921. [Google Scholar] [CrossRef]
- Pinazo, A.; Lozano, N.; Pe’rez, L.; Morán, M.C.; Infante, M.R.; Pons, R. Arginine diacyl-glycerolipid conjugates as multifunctional biocompatible surfactants. Comptes Rendus Chim. 2011, 14, 726–735. [Google Scholar] [CrossRef]
- Pérez, L.; Pons, R.; Oliveira de Sousa, F.F.; Morán, M.C.; Ramos da Silva, A.; Pinazo, A. Green cationic arginine surfactants: Influence of the polar head cationic character on the self-aggregation and biological properties. J. Mol. Liq. 2021, 339, 116819. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 4, 9–27. [Google Scholar] [CrossRef]
- Theoret, C. Physiology of Wound Healing. In Equine Wound Management, 3rd ed.; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–13. [Google Scholar] [CrossRef]
- Tavares, W.S.; Martin-Pastor, M.; Pérez, L.; Morán, M.C.; Sousa, F.F.O. Skin repairing potential of ellagic acid-loaded zein nanoparticles: Chemical and biopharmaceutical characterization, enzymatic inhibition and cytotoxicity over keratinocytes. J. Mol. Liq. 2023, 384, 122198. [Google Scholar] [CrossRef]
- Sgariglia, M.A.; Soberón, J.R.; Cabanes, A.P.; Sampietro, D.A.; Vattuone, M.A. Anti-inflammatory properties of phenolic lactones isolated from Caesalpinia paraguariensis stem bark. J. Ethnopharmacol. 2013, 147, 63–73. [Google Scholar] [CrossRef]
- Maksimenko, A.V.; Sakharova, Y.S.; Beabealashvilli, R.S. Experimental and Computational Study of Hyaluronidase Interactions with Glycosaminoglycans and their Ligands. Curr. Mol. Med. 2022, 22, 675–690. [Google Scholar] [CrossRef]
- Payan, E.; Jouzeau, J.Y.; Lapicque, F.; Muller, N.; Netter, P. Hyaluronidase degradation of hyaluronic acid from different sources: Influence of the hydrolysis conditions on the production and the relative proportions of tetra-and hexasaccharide produced. Int. J. Biochem. 1993, 25, 325–329. [Google Scholar] [CrossRef]
- Pérez, L.; Torres, J.L.; Manresa, A.; Solans, C.; Infante, M.R. Synthesis, Aggregation and Biological Properties of a New Class of Gemini Cationic Compounds from Arginine, bis(Args). Langmuir 1996, 12, 5296. [Google Scholar] [CrossRef]
- Suntar, A.I.; Altun, L.; KeLes, H.; Akkol, E.K. Phytochemical and biological studies on Alnus glutinosa subsp. glutinosa, A. orientalis var. orientalis and A. orientalis var. pubescens leaves. J. Ethnopharmacol. 2016, 192, 148–160. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, S.M. ChemBioOffice Ultra 2010 suite. J. Am. Chem. Soc. 2010, 132, 2466–2467. [Google Scholar] [CrossRef] [PubMed]










| Compound | Length of Spacer Chain | pKap | CMC (mM) Conductivity | CMC (mM) Fluorescence | CMC (mM) Surface Tension | γcmc mN/m |
|---|---|---|---|---|---|---|
| C3(OA)2 | 3 | n.d. | n.d. | 8 * | n.d. | n.d. |
| C3(CA)2 | 3 | n.d. | 2 * | 3 * | 0.043 * | 32 * |
| C3 (LA)2 OH | 3 | 10.9 | 0.6 * | 0.6 * | n.d. | n.d. |
| C3 (LA)2 | 3 | 11.4 | 0.5 * | 0.4 * | 0.005 * | 35 * |
| C6 (LA)2 | 6 | 10.5 | 0.4 * | 0.4 * | 0.002 * | 30 * |
| C9 (LA)2 | 9 | 9.4 | 0.3 * | 0.3 * | 0.003 * | 34 * |
| Compounds | Docking Score (kcal/mol) | Ligand | Receptor Pocket | Interaction Types | Distance (Å) | |
|---|---|---|---|---|---|---|
| Collagenase | C3(LA)2 | −12 | O(C=O) | GLU254 | Hydrogen Bond | 2.56398 |
| O(C=O) | ASN253 | Hydrogen Bond | 3.24822 | |||
| H(CH2) | TRP252 | Hydrogen Bond | 3.45238 | |||
| C6(LA)2 | −10 | N(NH) | GLU159 | Electrostatic | 4.30283 | |
| H(NH) | GLU1 | Hydrogen Bond | 2.05525 | |||
| H(NH Guanidine) | SER208 | Hydrogen Bond | 2.24523 | |||
| H(NH Guanidine) | GLU159 | Hydrogen Bond | 1.97905 | |||
| C9(LA)2 | −11.8 | N(NH) | GLU201 | Electrostatic | 4.75159 | |
| H(NH) | SER208 | Hydrogen Bond | 2.39595 | |||
| CH3 | TRP234 | Hydrophobic | 4.32331 | |||
| CH3 | TRP234 | Hydrophobic | 4.92808 | |||
| CH3 | TYR235 | Hydrophobic | 5.06206 | |||
| EGCG | −10.9 | O(OH) | SER423 | Hydrogen Bond | 2.78691 | |
| O(ROR) | THR438 | Hydrogen Bond | 2.99005 | |||
| H(OH) | THR7 | Hydrogen Bond | 2.20889 | |||
| Ring | GLU8 | Hydrogen Bond | 2.3916 | |||
| O(ROR) | GLY427 | Hydrogen Bond | 3.02574 | |||
| Ring | ASP424 | Electrostatic | 4.72785 | |||
| Ring | GLU1 | Electrostatic | 4.21691 |
| Compounds | Docking Score (kcal/mol) | Ligand | Receptor Pocket | Interaction Types | Distance (Å) | |
|---|---|---|---|---|---|---|
| Elastase | C3(LA)2 | −12.8 | H(NH) | ASP63 | Electrostatic | 4.22808 |
| H(NH Guanidine) | HIS60 | Hydrogen Bond | 2.64398 | |||
| CH3 | LEU156 | Hydrophobic | 3.95092 | |||
| C6(LA)2 | −10.9 | N(NH) | ASP63 | Electrostatic | 4.06755 | |
| O(C=O) | THR182 | Hydrogen Bond | 2.79448 | |||
| O(C=O) | VAL224 | Hydrogen Bond | 2.72013 | |||
| H(NH) | HIS60 | Hydrogen Bond | 3.00088 | |||
| H(NH Guanidine) | HIS60 | Hydrogen Bond | 1.92249 | |||
| CH3 | LEU66 | Hydrophobic | 5.08936 | |||
| CH3 | TYR35 | Hydrophobic | 4.15468 | |||
| C9C12 | −9.9 | O(C=O) | HIS60 | Hydrogen Bond | 2.67117 | |
| O(C=O) | THR182 | Hydrogen Bond | 2.12153 | |||
| H(NH Guanidine) | THR182 | Hydrogen Bond | 2.91942 | |||
| CH3 | LEU66 | Hydrophobic | 4.47598 | |||
| CH3 | TYR35 | Hydrophobic | 5.20529 | |||
| EGCG | −9.2 | H(OH) | HIS60 | Hydrogen Bond | 2.25921 | |
| O(C=O) | SER203 | Hydrogen Bond | 2.43056 | |||
| O(OH) | VAL224 | Hydrogen Bond | 2.01638 | |||
| O(C=O) | HIS60 | Hydrogen Bond | 2.79502 | |||
| H(OH) | THR44 | Hydrogen Bond | 2.19728 | |||
| Ring | HIS60 | Electrostatic | 4.57072 | |||
| Ring | CYS45 | Other | 5.93844 | |||
| Ring | HIS60 | Hydrophobic | 4.66195 |
| Compounds | Docking Score (kcal/mol) | Ligand | Receptor Pocket | Interaction Types | Distance (Å) | |
|---|---|---|---|---|---|---|
| Hyaluronidase | C3(LA)2 | −12.3 | N(NH) | GLU113 | Electrostatic | 3.80065 |
| O(C=O) | ARG47 | Hydrogen Bond | 2.30683 | |||
| O(C=O) | GLU113 | Hydrogen Bond | 2.79755 | |||
| CH3 | HIS23 | Hydrophobic | 5.49822 | |||
| C6(LA)2 | −11.8 | H(NH) | GLU113 | Hydrogen Bond; Electrostatic | 2.79842 | |
| O(C=O) | ARG47 | Hydrogen Bond | 2.20476 | |||
| O(C=O) | ARG47 | Hydrogen Bond | 2.69873 | |||
| O(C=O) | SER304 | Hydrogen Bond | 1.76159 | |||
| H(NH Guanidine) | GLN271 | Hydrogen Bond | 2.82288 | |||
| C9(LA)2 | −12 | H(NH) | GLU113 | Electrostatic | 4.05683 | |
| O(C=O) | ASN97 | Hydrogen Bond | 2.52287 | |||
| O(C=O) | ASN97 | Hydrogen Bond | 2.56576 | |||
| O(C=O) | GLU113 | Hydrogen Bond | 2.62381 | |||
| H(NH Guanidine) | TYR184 | Hydrogen Bond | 2.78541 | |||
| H(NH Guanidine) | GLN271 | Hydrogen Bond | 2.40937 | |||
| EGCG | −9.7 | O(C=O) | THR193 | Hydrogen Bond | 2.97624 | |
| O(C-O-C) | THR193 | Hydrogen Bond | 2.60255 | |||
| O(OH) | GLN271 | Hydrogen Bond | 2.60537 | |||
| O(OH) | EU192 | Hydrogen Bond | 2.37614 | |||
| O(C=O) | TYR190 | Hydrogen Bond | 3.51835 | |||
| O(OH) | SER225 | Hydrogen Bond | 3.6589 | |||
| Ring | ARG229 | Electrostatic | 4.45853 | |||
| Ring | ARG244 | Electrostatic | 3.70196 | |||
| Ring | PRO194 | Hydrophobic | 5.22577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, F.F.O.d.; Pinazo, A.; Hafidi, Z.; García, M.T.; Bautista, E.; Moran, M.d.C.; Pérez, L. Arginine Gemini-Based Surfactants for Antimicrobial and Antibiofilm Applications: Molecular Interactions, Skin-Related Anti-Enzymatic Activity and Cytotoxicity. Molecules 2023, 28, 6570. https://doi.org/10.3390/molecules28186570
Sousa FFOd, Pinazo A, Hafidi Z, García MT, Bautista E, Moran MdC, Pérez L. Arginine Gemini-Based Surfactants for Antimicrobial and Antibiofilm Applications: Molecular Interactions, Skin-Related Anti-Enzymatic Activity and Cytotoxicity. Molecules. 2023; 28(18):6570. https://doi.org/10.3390/molecules28186570
Chicago/Turabian StyleSousa, Francisco Fábio Oliveira de, Aurora Pinazo, Zakaria Hafidi, María Teresa García, Elena Bautista, Maria del Carmen Moran, and Lourdes Pérez. 2023. "Arginine Gemini-Based Surfactants for Antimicrobial and Antibiofilm Applications: Molecular Interactions, Skin-Related Anti-Enzymatic Activity and Cytotoxicity" Molecules 28, no. 18: 6570. https://doi.org/10.3390/molecules28186570
APA StyleSousa, F. F. O. d., Pinazo, A., Hafidi, Z., García, M. T., Bautista, E., Moran, M. d. C., & Pérez, L. (2023). Arginine Gemini-Based Surfactants for Antimicrobial and Antibiofilm Applications: Molecular Interactions, Skin-Related Anti-Enzymatic Activity and Cytotoxicity. Molecules, 28(18), 6570. https://doi.org/10.3390/molecules28186570